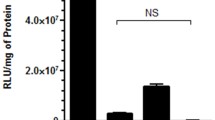
Abstract
Purpose
siRNA may be delivered as electrostatic complexes with cationic lipids (lipoplexes) or polycations (polyplexes). The purpose of this project was to determine the effect of cellular internalization mechanism(s) on siRNA-mediated gene silencing efficiency.
Methods
Lipoplexes were formed comprising siRNA and N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP), cholesterol and dioleoyl phosphatidylethanolamine (DOPE), and polyplexes comprised siRNA with polyethylenimine (PEI). During transfections, specific uptake mechanisms were inhibited by pharmacological agents and RNAi-mediated knockdown of proteins involved in various endocytosis pathways. Confocal fluorescence microscopy further elucidated the predominant endocytic pathways of siRNA delivery via colocalization of vectors with endocytic vesicle markers.
Results
Inhibition of macropinocytosis (MP), caveolin-mediated endocytosis (CvME), flotillin-mediated endocytosis (FME) and knockdown of ARF6 significantly decreased PEI/siRNA-mediated gene silencing. Inhibition of endocytosis pathways, however, had negligible effect on lipoplex uptake and gene silencing mediated by lipoplexes. Rather, internalization of lipoplexes and subsequent siRNA-mediated gene silencing occurred via an energy-independent process.
Conclusions
MP, CvME and FME, but not the acidified clathrin-mediated pathway, lead to effective gene silencing by PEI/siRNA polyplexes. Lipoplexes, in contrast, deliver siRNA primarily by direct fusion of the liposomal and cellular membranes. These results provide a new understanding of the mechanisms of siRNA delivery materials in HeLa cells and may aid in design of more effective RNAi strategies.






Similar content being viewed by others
Abbreviations
- ADE:
-
ARF6-dependent endocytosis
- ARF6:
-
ADP ribosylation factor 6
- CME:
-
Clathrin-mediated endocytosis
- CvME:
-
Caveolin-mediated endocytosis
- DOPE:
-
Dioleoyl phosphatidylethanolamine
- DOTAP:
-
N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate
- FME:
-
Flotillin-mediated endocytosis
- MP:
-
Macropinocytosis
- mβCD:
-
Methyl-β-cyclodextrin
- PEI:
-
Polyethylenimine
- RISC:
-
RNA-induced silencing complex
- RNAi:
-
RNA interference
- siRNA:
-
Small interfering RNA
References
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–8.
Fire A, Albertson D, Harrison SW, Moerman DG. Production of antisense RNA leads to effective and specific-inhibition of gene-expression in c-elegans muscle. Development. 1991;113(2):503–14.
Davidson BL, McCray PB. Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12(5):329–40.
Malefyt AP, Angart PA, Chan C, Walton SP. SiRNA therapeutic design: tools and challenges. In: Mallick B, Ghosh Z, editors. Regulatory RNAs: basics, methods and applications. Berlin: Springer; 2012. p. 475–503.
Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, et al. Purified argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12(4):340–9.
van de Water FM, Boerman OC, Wouterse AC, Peters JGP, Russel FGM, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34(8):1393–7.
Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10(5):766–71.
Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–8.
Gary DJ, Puri N, Won YY. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release. 2007;121(1–2):64–73.
Gao K, Huang L. Nonviral methods for siRNA delivery. Mol Pharm. 2009;6(3):651–8.
Frohlich T, Wagner E. Peptide- and polymer-based delivery of therapeutic RNA. Soft Matter. 2010;6(2):226–34.
Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902.
Kumari S, Swetha MG, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20(3):256–75.
Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5(11):424–8.
Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9(8):639–49.
Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10(4):364–71.
Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45.
Xiang SN, Tong HJ, Shi Q, Fernandes JC, Jin T, Dai KR, et al. Uptake mechanisms of non-viral gene delivery. J Control Release. 2012;158(3):371–8.
Reilly MJ, Larsen JD, Sullivan MO. Polyplexes traffic through caveolae to the golgi and endoplasmic reticulum en route to the nucleus. Mol Pharm. 2012;9(5):1280–90.
Payne CK, Jones SA, Chen C, Zhuang XW. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8(4):389–401.
Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8(1):46–U16.
Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278(43):41573–6.
Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3′-kinase-dependent machinery. Mol Biol Cell. 2006;17(8):3689–704.
Gabrielson NP, Pack DW. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J Control Release. 2009;136(1):54–61.
Hwang ME, Keswani RK, Pack DW. Dependence of PEI and PAMAM gene delivery on clathrin- and caveolin-dependent trafficking pathways. Pharm Res. 2015;32(6):2051–9.
Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12(3):468.
Goncalves C, Mennesson E, Fuchs R, Gorvel JP, Midoux P, Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol Ther. 2004;10(2):373–85.
von Gersdorff K, Sanders NN, Vandenbroucke R, De Smedt SC, Wagner E, Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol Ther. 2006;14(5):745–53.
Manunta M. Gene delivery by dendrimers operates via different pathways in different cells, but is enhanced by the presence of caveolin. J Immunol Methods. 2006;314(1–2):134–46.
van der Aa MAEM, Huth WS, Hafele SY, Schubert R, Oosting RS, Mastrobattista E, et al. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in Cos-7 cells. Pharm Res. 2007;24(8):1590–8.
Douglas KL. Toward development of artificial viruses for gene therapy: a comparative evaluation of viral and non-viral transfection. Biotechnol Prog. 2008;24(4):871–83.
Diaz-Moscoso A, Balbuena P, Gomez-Garcia M, Mellet CO, Benito JM, Le Gourrierec L, et al. Rational design of cationic cyclooligosaccharides as efficient gene delivery systems. Chem Commun. 2008;17:2001–3.
McLendon PM, Fichter KM, Reineke TM. Poly(glycoamidoamine) vehicles promote pDNA uptake through multiple routes and efficient gene expression via caveolae-mediated endocytosis. Mol Pharm. 2010;7(3):738–50.
Vercauteren D, Piest M, van der Aa LJ, Al Soraj M, Jones AT, Engbersen JFJ, et al. Flotillin-dependent endocytosis and a phagocytosis-like mechanism for cellular internalization of disulfide-based poly(amido amine)/DNA polyplexes. Biomaterials. 2011;32(11):3072–84.
Wang LJ, Rothberg KF, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–17.
Phonphok Y, Rosenthal KS. Stabilization of clathrin coated vesicles by amantadine, tromantadine and other hydrophobic amines. FEBS Lett. 1991;281(1–2):188–90.
Vercauteren D, Vandenbroucke R, Jones A, Rejman J, Demeester J, De Smedt S. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther. 2010;18(3):561–9.
Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161(4):673–7.
Koivusalo M, Welch C, Hayashi H, Scott C, Kim M, Alexander T. Amiloride inhibits macropinocytosis by lowering submembranous ph and preventing rac1 and cdc42 signaling. J Cell Biol. 2010;188(4):547–63.
Crook K, Stevenson BJ, Dubouchet M, Porteous DJ. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther. 1998;5(1):137–43.
Liu Y, Mounkes LC, Liggitt HD, Brown CS, Solodin I, Heath TD, et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol. 1997;15:167–73.
Pedroso de Lima MC, Simoes S, Pires P, Faneca H, Duzgunes N. Cationic lipid-DNA complexes in gene delivery: from biophysics to biological applications. Adv Drug Deliv Rev. 2001;47(2–3):277–94.
Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–57.
Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267(1):9–21.
Vonesch C, Unser M. A fast thresholded landweber algorithm for wavelet-regularized multidimensional deconvolution. IEEE Trans Image Process. 2008;17(4):539–49.
Larson DA. Analysis of variance with just summary statistics as input. Am Stat. 1992;46(2):151–4.
Wong LS. Elucidation of design criteria for siRNA delivery in mammalian cells using polyethylenimine. Ph.D. Thesis. University of Illinois, Urbana-Champaign; 2009.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69.
Li CX, Parker A, Menocal E, Xiang S, Borodyansky L, Fruehauf JH. Delivery of RNA interference. Cell Cycle. 2006;5(18):2103–9.
Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653–U119.
Chang K-L, Higuchi Y, Kawakami S, Yamashita F, Hashida M. Development of lysine–histidine dendron modified chitosan for improving transfection efficiency in HEK293 cells. J Control Release. 2011;156(2):195–202.
Chang K-L, Higuchi Y, Kawakami S, Yamashita F, Hashida M. Efficient gene transfection by histidine-modified chitosan through enhancement of endosomal escape. Bioconjug Chem. 2010;21(6):1087–95.
Pichon C, Billiet L, Midoux P. Chemical vectors for gene delivery: uptake and intracellular trafficking. Curr Opin Biotechnol. 2010;21(5):640–5.
Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102(1):247–53.
Lu JJ, Langer R, Chen JZ. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6(3):763–71.
Perez AP, Romero EL, Morilla MJ. Ethylendiamine core PAMAM dendrimers/siRNA complexes as in vitro silencing agents. Int J Pharm. 2009;380(1–2):189–200.
Juliano RL, Ming X, Nakagawa O. Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug Chem. 2012;23(2):147–57.
Pires P, Simoes S, Nir S, Gaspar R, Duzgunes N, de Lima MCP. Interaction of cationic liposomes and their DNA complexes with monocytic leukemia cells. Biochim Biophys Acta-Biomembr. 1999;1418(1):71–84.
Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413.
Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15–33.
Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608.
Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–500.
Ming X, Sato K, Juliano RL. Unconventional internalization mechanisms underlying functional delivery of antisense oligonucleotides via cationic lipoplexes and polyplexes. J Control Release. 2011;153(1):83–92.
Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol. 2011;22(1):18–26.
ACKNOWLEDGMENTS AND DISCLOSURES
We thank Sandy Mattick at the Cell Culture Media Facility at the University of Illinois for help with preparation of cell media. We also thank Maureen K. Thompson for assistance with cell culturing and flow cytometry experiments, Dr. B. Pilas for help with flow cytometry and Dr. M. Sivaguru for the help with microscopy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 8362 kb)
Rights and permissions
About this article
Cite this article
Lazebnik, M., Keswani, R.K. & Pack, D.W. Endocytic Transport of Polyplex and Lipoplex siRNA Vectors in HeLa Cells. Pharm Res 33, 2999–3011 (2016). https://doi.org/10.1007/s11095-016-2022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2022-1