Abstract
New complexes with formulae: Ln(4-bpy)(CBr2HCOO)3·3H2O (where Ln(III) = Gd, Tb, Dy) and Er(4-bpy)1.5(CBr2HCOO)3·3H2O, were prepared and characterized by chemical, elemental analysis and IR spectroscopy. The nature of metal–ligand coordination was studied. All of them are crystalline. Gd(III), Tb(III) and Dy(III) complexes are isostructural in one group. Conductivity studies (in methanol, dimethyloformamide and dimethylsulfoxide) were also discussed. The thermal properties of complexes in the solid state were studied using TG–DTG techniques under dynamic flowing air atmosphere. TG–MS system was used for Tb(III) (as an example of isostructural compound) and Er(III) complexes to analyze principal volatile thermal decomposition and fragmentation products evolved during pyrolysis in dynamic flowing air atmosphere.
Similar content being viewed by others
Introduction
Lanthanide compounds including N and O donors, as bipyridine isomers and carboxylate groups, are still the subject of current research. Based on the trend of lanthanides to create high coordination numbers and possibility of different coordination of selected organic ligands, it may be stated that such complexes can create a new class of compounds with potentially different functions and application. The mixed halogeno-bipyridine complexes of lanthanide(III) have been the subject of investigation for several years. The nature of bonds between Ln(III) ions and halogenoacetate ligand and bipyridine isomers is discussed in several papers [1–5]. These mixed ligand complexes are of interest because of possibility of various ways of coordination bipyridine and halogenoacetate ligands. These ligands are able to exist as mono- and multidentate donors in metal complexes [1–5]. In the literature [1, 2], there are described compounds, where there is only one type of coordination of carboxylate ligands. The papers [3–10] are examples to occur different possibilities of binding COO groups in one compound.
It is interesting to compare the properties of the mixed ligand lanthanide(III) complexes with 4,4′-bipyridine and chloro- or bromoacetates. These researches are recently undertaken in our laboratory.
Previously, the synthesis and characterization of lanthanide(III) complexes (where Ln(III) = Y, La → Lu without Pm(III)) with 4,4′-bipyridine and di- or trichloroacetates were reported [6–14]. This paper presents the results of new lanthanide complexes with title ligands. It is a continuation of our study on preparation, type of coordination organic ligands and thermal behavior of Y(4-bpy)1.5(CBr2HCOO)3·3H2O, Ln(4-bpy)(CBr2HCOO)3·nH2O where Ln(III) = La, Ce, Eu and Ln(4-bpy)0.5(CBr2HCOO)3·2H2O where Ln(III) = Pr, Nd, Sm complexes [14, 15].
Experimental
Materials, synthesis and analysis
4,4′-Bipyridine, CBr2HCOOH, Ln2O3 (where Ln(III) = Gd, Dy), Tb4O7, Er2O3, dimethylsulfoxide (DMSO), dimethylformamide (DMF) and methanol (MeOH) (anhydrous) p.a. were obtained from Aldrich and Lab-Scan, respectively. Water solutions of metal(III) dibromoacetates were prepared by adding 2 mol dm−3 dibromoacetic acid to freshly precipitated hydroxides in ca. stoichiometric quantities (in temperature ≤18 °C, because lanthanide dibromoacetate solutions are relatively unstable; in the presence of 4-bpy these stability increased).
The mixed ligand complexes were synthesized in the same way as we described in [15]. The contents of metal(III) ions in obtained solutions were mineralized and determined by EDTA titration.
The carbon, hydrogen and nitrogen contents in the prepared complexes were determined by a Carlo-Erba analyzer with V2O5 as an oxidizing agent.
Methods and instruments
The X-ray powder diffraction patterns of synthesized complexes and final solid decomposition products in air were recorded on D-5000 diffractometer using Ni-filtered CuKα radiation. The measurements were taken in the range of 2θ angles 2°–80°. Obtained results were analyzed using the Powder Diffraction File [16]. Molar conductance was measured on a conductivity meter of the OK-102/1 type equipped with an OK-902 electrode at 25 ± 0.5 °C, using 1 × 10−3 mol dm−3 solutions of complexes in methanol, dimethylformamide and dimethylsulfoxide. IR spectra were recorded with a NICOLET 6700 Spectrometer (4000–400 cm−1 with accuracy of recording 1 cm−1) using KBr pellets. The thermal properties of complexes in air were studied by TG–DTG techniques in the range of temperature 25–1000 °C at a heating rate of 10 °C min−1; TG and DTG curves were recorded on Netzsch TG 209 apparatus in flowing dynamic air atmosphere v = 20 cm3 min−1 using ceramic crucibles. The solid intermediate products of pyrolysis in air were determined from TG and DTG curves and confirmed by recording the IR spectra of sinters (prepared during heating the sample of complex up to appropriate temperature defined from the TG or DTG curves). The IR spectra of sinters were analyzed in the region characteristic for 4,4′-bipyridine and dibromoacetates absorption. Additionally, in the sinters, the presence of anions Br− was also investigated. The TG–MS coupled measurements have been carried out only for Tb(III) and Er(III) complexes using the SETSYS 16/18 apparatus coupled with QMS-422 Balzers spectrometer, in the range of temperature 25–1000 °C at a heating rate 10 °C min−1 in flowing air atmosphere v = 20 cm3 min−1 in ceramic crucibles. The m/z values are given based on 1H, 12C, 14N, 16O and 80Br (additionally 13C and 18O in case of CO2).
Results and discussion
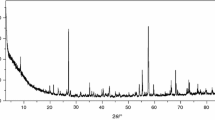
Table 1 presents results of the elemental and chemical analysis of investigated compounds. They are stable in air in solid state. The analysis of the power diffraction patterns of these compounds reveals that they are crystalline. Gd(III), Tb(III) and Dy(III) complexes are isostructural in one group (Fig. 1). The Er(III) compound is not isostructural with them. There are no powder diffraction patterns of reported complexes in Powder Diffraction File [16]. There were isolated monocrystals of Dy(III) compound, but they have very poor quality (not suitable data for publication). Also in Table 1, there are molar conductivity values for all complexes in solutions of MeOH, DMF and DMSO. All complexes in MeOH and DMF display behaviors intermediate between those of nonelectrolytes and 1:1 electrolytes. In DMSO they are electrolyte type 1:1 [17]. In series of light lanthanide complexes (Ce(III) → Eu(III)) with 4-bpy and dibromoacetates, the molar conductivities in solutions MeOH, DMF and DMSO have similar values. Relative not high molar conductance in MeOH and DMF is characteristic for all complexes compared. The conductivity data suggest that dibromoacetate ligands in obtained compounds are only partially displaced by solvent molecules [18, 19].
IR spectra
In Tables 2 and 3, there are absorption bands in the region characteristic for dibromoacetates and 4-bpy. These spectra are very similar. For all compounds, the νas(COO) vibration is identified as the strong band in the range 1712–1690 cm−1. The band assigned to the vibration of νs(COO) is observed in the range 1412–1385 cm−1. Only for Er(III) complex, νs(COO) vibrations are split into two. The vibrations of νas(COO) and νs(COO) are shifted to higher wave numbers. This fact may indicate that the carboxylate groups are bonded to metal(III) ions as bridging ligands [22–24]. For Er(III) compound, probably bridging non-completely equivalent bonds between Er(III) and carboxylate groups exists [25]. In the absorption region of CBr2HCOONa appear also the stretching modes νs(CBr2) and δ(CCOO). They are in complexes observed in the ranges: 700–720 cm−1 and between 1145 and 1140 cm−1, respectively. The absorption bands of ν(CH) are in the sodium salt at 3015 and 1191 cm−1; in the complexes, they are in the ranges: 3022–2986 and 1181–1186 cm−1.
In the IR spectra of complexes, there are also vibrations of 4-bpy. They are given in Table 3. The observations were made for 4-bpy absorption in the region 4000–600 cm−1. The characteristic band attributed to ν(CC), ν(CN), ν(CCir) vibrations (symmetry A 1 ) in pure 4-bpy is at 1588 cm−1, but in complexes, it is in the range 1615–1599 cm−1. The band in the free ligand at 1530 cm−1 assigned to ν(CC), ν(CC) vibrations (symmetry B 1 ) in the IR spectra of complexes, is slightly shifted to higher frequency. The band characteristic for “breathing” mode is moved to higher wave numbers (1003–1000 cm−1) in comparison with free ligand (988 cm−1). Most of aromatic ring deformation bands in plane γ(CH) and out plane β(CH) shift to lower and higher frequency after coordination with metal ion [21].
In addition, a broad band in the water stretching region (ca 3450–3350 cm−1) appears for all complexes described.
Thermal decomposition in air
The TG–DTG methods were used to describe thermal decomposition in air of synthesized complexes. First step of pyrolysis is dehydration. After that, partial decomposition and next total decomposition of organic ligands take place. Most processes are poorly separated from each other. In several intermediate solid products, presence of bromide, carboxylate group and bipyridine was identified. As a final, solid decomposition products are suitable lanthanide oxides. This has been proven on the basis of diffraction patterns (as an example Fig. 2) taken for final solid products. All synthesized compounds do not reveal the effect of creating LnOBr. Figures 3, 4, 5 6 show profiles of TG and DTG of all obtained complexes. Results of thermal analysis are presented in Table 4.
Gd(4-bpy)(CBr2HCOO)3·3H2O starts to decompose at 75 °C. In the temperature ranges: 75°–100° and 100–200 °C, two-stage dehydration takes place. First, Gd(III) complex loses one water molecule and next two moles of water. Anhydrous compound is stable up to 200 °C. When temperature rises, partial decomposition of dibromoacetates occurs. Probably intermediate solid product appears, Gd(4-bpy)(CBr2HCOO)2.75·Br0.25 (mass lose calculated 3.67 % and found 4.0 %). On DTG curve appears peak at 205 °C. Next (220–290 °C), there is further decomposition of halogenoacetates probably via Gd(4-bpy)(CBr2HCOO)·Br2 accompanied with strong maximum on DTG at 245 °C. Subsequent thermolysis of Gd(4-bpy)(CBr2HCOO)·Br2 is observed with DTG peaks at 330, 440 and 815 °C. Horizontal mass level for Gd2O3 begins at 835 °C.
Terbium(III) complex decomposes, also as Gd(III) compound, at 75 °C. First, it loses one and next two molecules of water. On DTG curve, there are two peaks at 80 and 135 °C. Above 155 °C, Tb(4-bpy)(CBr2HCOO)3 converts probably to Tb(4-bpy)(CBr2HCOO)2.5·Br0.5. It is accompanied with DTG peak at 200 °C. Further heating causes decomposition of first partial and then total organic ligands (DTG maximum at 245, 430 and 660 °C). These processes are poorly separated from each other. A plateau on TG curve for Tb4O7 begins above 750 °C (mass lose calculated 81.67 % and found 81.3 %).
Dy(III) compound is stable up to 60 °C. On the beginning, complex loses 1.5 mol of water. In the range of temperature 125–200 °C further dehydration and decomposition to Dy(4-bpy)(CBr2HCOO)2.75·Br0.25 take place (mass lose calculated 5.99 % and found 6.0 %). This is connected with DTG peak at 185 °C. Next (200–350 °C), Dy(4-bpy)(CBr2HCOO)0.5·Br2.5 appears. After that, further pyrolysis (destruction of dibromoacetate groups and oxidation of organic fragments) takes place by the overlap of multiple processes. The DTG curve shows peaks at 250° (with shoulder at 260°), 430 and 770 °C. At 800 °C, Dy2O3 forms.
Er(III) complex is stable up to 50 °C. Dehydration process occurs in two steps. Above 50 °C, one and half water molecules are lost (50–80 °C). The second step takes place between 80 and 145 °C. It is connected with leaving the remaining water. On DTG curve, there are peaks at 65° and 125° with shoulder at 150 °C. With increasing temperature is partial and total destroying of organic ligands probably via Er(4-bpy)1.5(CBr2HCOO)0.5·Br2.5. Above 775 °C, pure Er2O3 appears.
TG–MS study for Tb(III) and Er(III) complexes in air
Moreover, TG–MS system was used to analyze the principal volatile products evolved during thermal decomposition and fragmentation in dynamic air atmosphere. TG–MS study was made only for terbium(III) (as a representative of isostructural compounds) and erbium(III) complexes. The detection of volatile species and fragmentation in appropriate temperature range (270–370 °C for Tb(IIII) and 260–360 °C in case of Er(III)) correspond exactly to the sudden mass loss presented in Table 4. Major maxima for ion current are for Tb(III) at: 270, 450 and 680 °C. In case of Er(III) compound, they are at: 265, 455, 514 and 720 °C. The peak characteristic for OH+, H2O+ (m/z = 17, 18) appears as a broad maximum in the temperature range 90–320 °C. It connects with dehydration and partial decomposition dibromoacetate ligands. For terbium(III) complex, the profiles of C+ and CO2 + (m/z = 12, 44, 45, 46) are maximum at 180° and 233°, and with very high intensity at 280, 460, 517 and 694 °C. In case of the erbium(III) compound, they are at: 146° (with low intensity), 270, 455, 515 and 720 °C. For investigated complexes, the bromide species corresponding to m/z = 80 (Br+), 81 (HBr+), 93 (CHBr+), 94 (CH2Br+), 95 (CH3Br+), 174 (CH2Br +2 ) are in the temperature range 250–400 °C. It is associated with destruction of dibromoacetates. There are also fragments m/z = 15 (CH3 + or NH+) and 30 (CH2O+ or NO+). Maximum formation of CH3 + or NH+ is at 280 °C. Evolution of CH2O+ or NO+ is at 670 °C (Tb(III)) and 725 °C (Er(III)). The mass spectrometer detected also traces of other ion signals. Figure 7 presents hemogram of Tb(III) complex consisting of only principal bromide species versus time.
Conclusions
-
1.
In this paper, there is information about obtained complexes of Gd(III), Tb(III), Dy(III) and Er(III) with empirical formulae: Ln(4-bpy)(CBr2HCOO)3·3H2O (where Ln(III) = Gd, Tb, Dy) and Er(4-bpy)1.5(CBr2HCOO)3·3H2O. All compounds were synthesized in the same experimental conditions.
-
2.
The compounds of Gd(III), Tb(III) and Dy(III) are isostructural in one group.
None of the obtained complexes is isostructural of compounds of Y(III), La(III) → Eu(III) (without Pm(III)) with 4,4′-bipyridine and dibromoacetates [14, 15].
-
3.
The IR spectroscopy data for obtained Gd(III), Tb(III), Dy(III) and Er(III) compounds confirm that the metal ions(III) are bonded with title ligands. The shifts of bands characteristic for νas(COO) and νs(COO) vibrations in complexes correspond to bridging nature of carboxylate groups.
-
4.
During heating in air, all complexes decompose in multistage. Some stages of pyrolysis are weakly separated one from another. Decomposition of investigated compounds begins with total or partial loss of water. Elevation of temperature is connected with partial destruction of ligands containing bromides. The final solid residues are pure oxides.
-
5.
MS data for Tb(III) and Er(III) complexes detected several profiles of ion currents. The principal volatile products evolved during thermal decomposition and fragmentation processes in dynamic air atmosphere are: OH+, H2O+, C+, CO2 +, Br+, HBr+, CHBr+, CH2Br+, CH3Br+, CH2Br +2 , CH2O+ or NO+, CH3 + or NH+.
References
Weimin L, Yiqiang C, Nan D, Jianming G, Chenggang C. Synthesis, characterization and crystal structure of diaquadi(2,2′-bipyridine)di(dichloroacetato)lanthanide(III) monodichloroacetato. J Coord Chem. 1994;35:51. doi:10.1080/00958979508033085.
Rohde A, John D, Urland W. Crystal structures of Gd2(Cl3CCOO)6(bipy)2(H2O)2·4-bipy, Pr(Cl3CCOO)3(bipy)2, Nd(Cl3CCOO)3(bipy)2 and Er(Cl3CCOO)3(bipy)2(H2O). Z Kristal. 2005;220(2):177. doi:10.1524/zkri.220.2.177.59141.
John D, Urland W. Crystal structure and magnetic behaviour of the new gadolinium complex compound Gd2(ClH2CCOO)6(bipy)2. Eur J Inorg Chem. 2005;22:4486. doi:10.1002/ejic.200500734.
Rohde A, Urland J. Catena-Poly[[(2,2′-bipyridine-κ2N, N′)-praseodymium(III)]-μ-dichloroacetate-1κ2O:O′:2κO-di-μ-dichloroacetato-κ4O:O′]. Acta Cryst. 2006;E62:2843. doi:10.1107/S160053680603995X.
Rohde A, Urland J. Catena-Poly[[(2,2′-bipyridine-κ2N, N′)-neodymium(III)]-μ-dichloroacetate-1κ2O:O′:2κO-di-μ-dichloroacetato-κ4O:O′]. Acta Cryst. 2006;E62:1618. doi:10.1107/S1600536806022872.
Czylkowska A, Kruszynski R, Czakis-Sulikowska D, Markiewicz M. Coordination polymer of lanthanum: synthesis, properties and crystal structure of [La(4,4′-bipyridine)(CCl2HCOO)3(H2O)]n. J Coord Chem. 2007;60(24):2659–69.
Kruszyński R, Czylkowska A, Czakis-Sulikowska D. A novel carboxylic coordination polymer of samarium(III): [Sm(H2O)(4,4′-bipyridine)(CCl2HCOO)3]n. J Coord Chem. 2006;59(6):681–90.
Czylkowska A, Markiewicz M. Coordination behaviour and thermolysis of some rare-earth complexes with 4,4′-bipyridine and di- or trichloroacetates. J Therm Anal Calorim. 2010;100(2):717–23.
Czylkowska A, Czakis-Sulikowska D, Kruszynski R, Markiewicz M. Synthesis, crystal structure and other properties of the complexes of Er(III), Yb(III) and Lu(III) with 4,4′-bipyridine and dichloroacetates. Struct Chem. 2010;21(2):415–23.
Czylkowska A, Czakis-Sulikowska D, Kaczmarek A, Markiewicz M. Thermal behavior and other properties of Pr(III), Sm(III), Eu(III), Gd(III), Tb(III) complexes with 4,4′-bipyridine and trichloroacetates. J Therm Anal Calorim. 2011;105(1):331–9.
Czakis-Sulikowska D, Czylkowska A, Markiewicz M. Synthesis, characterization and thermal decomposition of yttrium and light lanthanides with 4,4′-bipyridine and dichloroacetates. Polish J Chem. 2007;81(7):1267–75.
Czakis-Sulikowska D, Czylkowska A, Markiewicz M, Frajtak M. Synthesis and properties of complexes of Gd(III), Tb(III), Ho(III) and Tm(III) with 4,4′-bipyridine and dichloroacetates. Polish J Chem. 2009;83(11):1893–901.
Czylkowska A. New complexes of heavy lanthanides with 4,4′-bipyridine and trichloroacetates. J Therm Anal Calorim. 2011;105(1):331–9.
Czylkowska A, Markiewicz M. Synthesis, thermal behavior, and other properties of Y(III) and La(III) complexes with 4,4′-bipyridine and trichloro-or dibromoacetates. J Therm Anal Calorim. 2012;109(2):727–34.
Czylkowska A. Synthesis and some properties of light lanthanide complexes with 4,4′-bipyridine and dibromoacetates: thermal study. J Therm Anal Calorim. 2013;114:989.
Powder Diffraction File, PDF-2. The International Centre for Diffraction Data (ICDD) 12 Campus Boulevard, Newton Square, PA, USA 2004.
Geary WI. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev. 1971;7:81–122. doi:10.1016/S0010-8545(00)80009-0.
Harris CM, McKenzie ED. Nitrogenous chelate complexes of transition metals.III. Bis-chelate complexes of nickel(III) with 1,10-phenantroline, 2,2′-bipyridyl and analogous ligands. J Inorg Nucl Chem. 1967;29(4):1047–68.
Feltman RD, Hayter RG. The electrolyte type of ionized complexes. J Chem Soc 1964;4587–91.
Spinner E. The vibration spectra of some substituted acetate ions. J Chem Soc 1964;4217.
Pearce CK, Grosse DW, Hessel W. Effect of molecular structure on infrared spectra of six isomers of bipyridine. Chem Eng Data. 1970;15:567–70. doi:10.1021/je60047a042.
Manhas BS, Trikha AK. Relationships between the direction of shifts in the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Indian J Chem. 1982;59:315–9.
Liu J-Y, Ren N, Zhang J-J, He S-M, Wang S-P. Crystal structures, thermal properties, and biological activities of a series of lanthanide compounds with 2,4-dichlorobenzoic acid and 1,10-phenanthroline. Ind Eng Chem Res. 2013;52:6156–63.
Zhang Y-Y, Ren N, Xu S-L, Zhang J-J, Zhang D-H. A series of binuclear lanthanide(III) complexes: crystallography, antimicrobial activity and thermochemistry properties studies. J Mol Struct. 2015;1081:413–25.
Brzyska W, Ożga W. Spectral, magnetic and thermal investigations of some d-electron element 3-methoxy-4-methylbenzoates. J Therm Anal Calorim. 2006;84:385–9. doi:10.1007/s10973-005-6855-9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czylkowska, A. Synthesis, thermal study and some properties of Gd(III), Tb(III), Dy(III) and Er(III) complexes with 4,4′-bipyridine and dibromoacetates. J Therm Anal Calorim 122, 339–347 (2015). https://doi.org/10.1007/s10973-015-4673-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4673-2