Abstract
New complexes with formulae: Zn(CClH2COO)2·2H2O, Zn(CCl2HCOO)2·2H2O, Zn(CCl3COO)2·2H2O, Cd(CCl2HCOO)2·H2O, Cd(CCl3COO)2·2H2O and Pb(CCl3COO)2·2H2O, were prepared and characterized by chemical, elemental analysis and IR spectroscopy. The nature of metal–ligand coordination was studied. They are small crystalline. The thermal properties of compounds in the solid state were studied using TG–DTG techniques under dynamic flowing air atmosphere. Mass spectrometer was used to analyze principal volatile thermal decomposition and fragmentation products of Zn(CCl3COO)2·2H2O evolved during pyrolysis in dynamic flowing air and in argon atmosphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The carboxylic acids are one of the most important organic acids because of their application. They are starting compounds for the synthesis of many derivatives. Substituents of the carboxylic acids have an impact on acidity. A carboxylate group as a ligand can coordinate with metal ion in various ways [1], making it interesting. The title chloroacetic acid is widely used. It is used in medicine since it has astringent and antiseptic properties, in the medical analysis to remove proteins from blood or serum in the determination of glucose. Sodium salt of trichloroacetic acid is used in agriculture, as one of the herbicide. It is in the fourth class of compounds known as damaging agents. In the plastics industry, it is used as a solvent for plastics, in metal industry to the surface treatment of metals, due to of the corrosive properties [2, 3]. This acid is widely present in the environment. It is characterized by very high stability. Their high solubility in water makes, that it occurs in the rain, snows, ice, fog fresh and marine waters as well as in the soil [4, 5].
To the research were selected d-block metals and lead (although it is metal of p-block). Lead and zinc are classified as non-ferrous metals. A common feature of the selected elements is the ability to create different type of complexes.
In literature there is a note about Zn(II) chloroacetate and its complexes with nicotinamide and caffeine [6]. Also, there is some information about Zn(II), Cd(II) and Pb(II) with acetic acids [7–18].
This paper investigates several solid compounds of Zn(II) and Cd(II) with mono-, di-, or trichloroacetic acids and Pb(II) with trichloroacetic acids (as the most common compound of lead with chloroacetates, which is in the soil). Their stoichiometric composition, nature of the metal–organic ligand bond, molar conductivity in various solvents and thermal behavior were studied. Thermal stability in air, information about stages of pyrolysis, intermediate and final solid products of decomposition of investigated compounds are described. In addition, for Zn(CCl3COO)2·2H2O as an example, volatile species emitted during thermal decomposition in air and for comparison in argon atmosphere were studied. This compound was chosen because it is important for the environment.
Experimental
Materials, synthesis and analysis
CClH2COOH, CCl2HCOOH and CCl3COOH were obtained from Aldrich. Zinc(II) monochloroacetates, zinc(II) dichloroacetates, zinc(II) trichloroacetates, cadmium(II) dichloroacetes, cadmium(II) trichloroacetates and lead(II) trichloroacetates were prepared by adding 2 mol L−1 mono-, di- or trichloroacetic acid to freshly precipitated metal(II) carbonates (in room temperature). After obtaining saturated solutions, the excess of carbonates was filtered off. These solutions were left to crystallize.
The contents of C and H in prepared compounds were determinate by a Carlo-Erba analyzer with V2O5 as an oxidizing agent; the metal(II) ions in obtained solutions were determined by EDTA titration.
Methods and instruments
IR spectra were recorded with a NICOLETT 6700 Spectrometer (4000–400 cm−1 with accuracy of recording 1 cm−1) using KBr pellets. Molar conductance was measured on a conductivity meter of the OK-102/1 type equipped with an OK-902 electrode at 25 ± 0.5 °C, using 1 × 10−3 mol L−1 solutions of complexes in methanol, dimethylformamide and dimethylsulfoxide. The thermal properties of complexes in air were studied by TG–DTG techniques in the range of temperature 25–1000 °C at a heating rate of 10 °C min−1; TG and DTG curves were recorded on Netzsch TG 209 apparatus in flowing dynamic air atmosphere v = 20 mL min−1 using ceramic crucibles. The mass spectra were measured on mass spectrometer ThermoStar, Balzers (Germany), in the range of temperature 25–1000 °C at a heating rate 10 °C min−1 in flowing air and argon atmosphere v = 20 mL min−1 in ceramic crucibles. The m/z values are given based on 1H, 12C, 16O and 35Cl (additionally 13C and 18O in case of CO2), in the range m/z: 1–120. The X-ray powder diffraction patterns of synthesized complexes and final solid decomposition products in air were recorded on D-5000 diffractometer using Ni-filtered CuKα radiation. The measurements were carried out in the range of 2θ angles 2° – 80°. Obtained results were analyzed using the Powder Diffraction File [19].
Results and discussion
In the solid state, six new compounds with formulae: Zn(CClH2COO)2·2H2O, Zn(CCl2HCOO)2·2H2O, Zn(CCl3COO)2·2H2O, Cd(CCl2HCOO)2·H2O, Cd(CCl3COO)2·2H2O and Pb(CCl3COO)2·2H2O were obtained. Unfortunately, obtained Cd(II) compound with monochloroacetates has heterogeneous composition. Therefore, further research was discontinued.
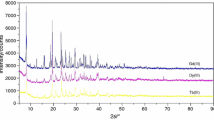
Table 1 presents results of the elemental and chemical analysis of isolated compounds. They do not change their stoichiometric composition in solid state. The analysis of the power diffraction patterns of these compounds shows that they are small crystalline. The most crystalline is Pb(CCl3COO)2·2H2O. Figure 1 presents, as an example, X-ray diffraction pattern of lead(II) compound. Table 1 shows molar conductivities values for all investigated compounds in solutions: MeOH, DMF and DMSO. All of them in DMF and also Zn(CCl3COO)2·2H2O and Pb(CCl3COO)2·2H2O in DMSO fall within the generally acceptable range for non-electrolytes [20]. All compounds in MeOH and Zn(CClH2COO)2·2H2O, Zn(CCl2HCOO)2·2H2O, Cd(CCl2HCOO)2·H2O, Cd(CCl3COO)2·2H2O in DMSO display behaviors intermediate between those of non-electrolytes and 1:1 electrolytes. Relatively not high molar conductance shows that they dissociate only in limited degree in these solutions.
IR spectra
Tables 2–4 show absorption bands in the region characteristic for mono- di- and trichloroacetates. For all compounds, the vibration of ν as(COO) and ν s(COO) are identified as the strong bands. The criterions of possible coordination of carboxylate groups in obtained compounds are determined based on the separation parameter Δν = ν as(COO)–ν s(COO) [22–24]. In the investigated compounds, the values of Δν (Δν < Δν Na) indicate mainly bidentate chelating type of coordination of carboxylate groups.
In all cases, the bands ν s(COO) are split into two. Additionally, for Zn(CCl2HCOO)2·2H2O and Cd(CCl3COO)2·2H2O compounds appears also separation of ν as(COO) band. Based on these data, we can conclude about the different coordination of organic ligands in one compounds, as well as, about formation of non-completely equivalent bands between metal(II)-carboxylate groups [25, 26].
In addition, a broad band in the water stretching region (ca 3450–3350 cm−1) appear for all described complexes.
Thermal decomposition
Thermal decomposition of analyzed compounds in air is a multistage process. The solid intermediate products of pyrolysis were determined from TG and DTG curves. The thermal decomposition data are collected in Table 5. Figures 2–5 present the thermoanalytical curves of obtained complexes. From TG and DTG curves the solid intermediate products of decomposition were determined. They were also confirmed by the IR spectra of sinters. In the sinters (prepared during heating of complexes up to temperatures defined from TG curves), the vibration modes of chloroacetates were analyzed as well as the presence of anions Cl− was also stated. All Zn(II) and Cd(II) compounds are stable up to 50 °C. Only Pb(CCl3COO)2·2H2O starts to decompose at 100 °C.
The compound of Zn(CClH2COO)2·2H2O loses 0.5 mol of water in a first stage of decomposition (50–125 °C); mass founded 2.70% and calculated 3.12%. Further dehydration process is accompanied by partial destruction of monochloroacetates. In the ranges of temperature: 125–300 and 300–340 °C intermediate species Zn(CClH2COO)0.5Cl1.5 and Zn(CClH2COO)0.25Cl1.75 are created. Next, total decomposition of monochloroacetates takes place. Between 340 and 525 °C high mass loss appears (42.30%), which is probably associated with the formation of volatile decomposition products containing zinc.
Dehydration of Zn(CCl2HCOO)2·2H2O is in two steps. In the range 50–175 °C one and half water molecules are released. Zn(CCl2HCOO)2·0.5H2O species are stable up to 175 °C. When the temperature rises on TG curves is observed the biggest mass loss (found 48.00%, calculated 47.82%), which is associated with further dehydration and partial combustion of organic ligands (175–275 °C). In the temperature range 325–500 °C volatile products containing zinc appear.
The Cd(CCl2HCOO)2·H2O losses 1 mol of water above 50 °C. Next, in the interval 140–200 °C partial destruction of dichloroacetates takes place and forms intermediate specie type Cd(CCl2HCOO)0.5Cl1.5. The TG curve shows a rapid mass loss between 200 and 240 °C as a result of total decomposition of organic ligands (CdCl2 is formed). In the range 240–660 °C CdO is created. The mass loss calculated is 14.21%, but founded is 45.00%. This is probably related with the formation of volatile products with cadmium.
Thermolysis of Zn(CCl3COO)2·2H2O starts with the dehydration. Zinc compound losses two water molecules in one stage (50–90 °C). When the temperature rises, decomposition of organic ligands appears and ZnCl2 exists. It is accompanied by a high mass loss on TG curve (found 59.50%, calculated 59.65%). When the temperature rises (350–475 °C) volatile products containing zinc are formed.
Thermal decomposition of Cd(CCl3COO)2·2H2O begins at 50 °C. On TG curve is recorded a high mass loss associate with dehydration and partial destruction of organic ligands (intermediate specie Cd(CCl3COO)Cl is formed). Increasing temperature causes further degradation and creates CdCl2 (145–190 °C). After this process volatile products containing cadmium are emitted. Horizontal mass level for CdO starts above 650 °C.
Compound of Pb(II) starts to decompose at 100 °C. On TG curve appears rapid mass loss (found 39.50%, calculated 39.86%). Such a decrease in mass is linked with dehydration, and partial decomposition of carboxylates, Pb(CCl3COO)0.5Cl1.5, occurs. Above 180 °C PbO forms.
MS study
Mass study was used to analyze the principal volatile products evolved during the thermal decomposition and fragmentation processes only for Zn(CCl3COO)2·2H2O compound. The determination was carried out in dynamic air and argon atmospheres.
MS study in air
For Zn(CCl3COO)2·2H2O major maxima for ion currents are observed in temperature range 190–220 °C. For OH+ and H2O+ (m/z = 17, 18), there is one peak around 85 °C which is connected with the dehydration of zinc(II) compound. When temperature rises, several gaseous products start to liberate. The profiles of CO2 + (m/z = 44, 45) exhibit very intensity maximum at 195 °C. At the same temperature is also emitted C+ with m/z = 12. These ion signals (C+, CO2 +) also have the same intensity and similar profiles. The mass spectrometer detected also many fragments containing chlorine with m/z = 35 (Cl+), 47 (CCl+), 70 (CCl2 +), 82 (CCl2 +), 117 (CCl3 +). The chlorine species appear between 190 and 220 °C. These molecular ions have the main peaks in the temperature close to evolution of carbon and dioxide. This proves a one-step destruction of trichloroacetates. Further small peaks of gaseous products containing chlorine (very low intensities of ion currents) indicate the combustion of organic ligands. The evaluation of all gaseous products finishes at ca 600 °C. Figure 6 presents as an example some ion currents vs temperature.
MS study in argon
In inert atmosphere the ion currents appear in the range 200–240 °C. The peaks of H2O+ (m/z = 18) and OH+ (m/z = 17) are connected with dehydration and occur at about 200 °C. The ion signal intensities of CO+ (m/z = 28) and CO +2 (m/z = 44) have centers at 210 °C. The profile of C+ (m/z = 12) with very low intensity has a maximum at ca. 240 °C. These facts suggest decomposition and oxidization of organic ligands and the burning of the organic residues. The major ion signals containing chloride with m/z = 35, 47, 70 and 82 (Cl+, CCl+, Cl +2 , CCl +2 ) have centers between 200 and 240 °C. Additionally, the strong peaks of O+ and O +2 (m/z = 16 and 32) were monitored at 200 °C and at 220 °C, respectively. Thermolysis of Zn(CCl3COO)2·2H2O is one-stage in argon atmosphere. The process of dehydration is connected with the decomposition of organic ligands. It was confirmed by emission of gaseous products. As an example, Fig. 7 shows some ion currents vs temperature.
Conclusions
From the system: M(II)–RCOOH–H2O (where M(II) = Zn, Cd, Pb; RCOOH = CClH2COOH, CCl2HCOOH and CCl3COOH) six new compounds with stoichiometry: Zn(CClH2COO)2·2H2O, Zn(CCl2HCOO)2·2H2O, Zn(CCl3COO)2·2H2O, Cd(CCl2HCOO)2·H2O, Cd(CCl3COO)2·2H2O and Pb(CCl3COO)2·2H2O were obtained. Described in [6] compound of zinc with monochloroacetates also contains two water molecules. Most zinc and cadmium acetates were obtained as dihydrated compounds [7–10, 12, 13, 16]. Only Györyovà and Balek [17] synthesized zinc acetate with formulae Zn(CH3COO)2·2.5H2O.
The IR spectra of the synthesized compounds show, that all carboxylate groups from chloroacetate ligands coordinate with metal(II) atoms. Infrared spectra give us the information about various types of organic ligands bonds.
All obtained compounds are stable at room temperature. During heating they decompose progressively. They start to decompose at 50 °C (except Pb(II), which is stable up to 100 °C). When the temperature rises dehydration and decomposition of organic ligands take place. There are differences in thermal decomposition of zinc and cadmium acetates in comparison to zinc and cadmium chloroacetaes. These differences come from formation of various products containing chlorine. This is reflected in the solid final products of thermolysis [7–10, 12, 13, 16, 17].
In case of zinc(II) and cadmium(II) chloroacetates formation of volatile species containing metal(II) ions take place. A similar phenomenon has been described in the literature [8], where the authors recorded higher than expected final mass loss. It has been attributed the formation of volatile compounds containing metal(II).
Mass study was used to analyze the principal volatile thermal decomposition and fragmentation products in air and in argon of Zn(CCl3COO)2·2H2O. Relative, the most species are detected in temperature ranges 190–220 °C (in air) and 200–240 °C (in argon). In air and argon atmosphere were observed different levels of intensity evolving molecular ions with m/z = 12 and 44 (C+ and CO +2 ). In air was not observed presence of CO+. In both atmospheres some fragments containing chlorine (Cl+, CCl+, Cl +2 , CCl +2 and CCl +3 ) were detected. These molecular ions were not observed by the authors [6, 17].
The results described in this paper complete existing knowledge of metal(II) compounds with halogenoacetates.
References
Mikuriya M. Copper(II) acetate as a motif for metal-assembled complexes. Bull Jpn Soc Coord Chem. 2008;52:17–28.
Varshney M, Chandra A, Chauhan LKS, Goel SK. In vitro cytogenetic assessment of trichloroacetic acid in human peripheral blood lymphocytes. Environ Sci Pollut Res. 2014;21(2):843–50.
Heal MR, Dickey CA, Heal KV, Stidson RT, Matucha M, Cape JN. The production and degradation of trichloroacetic acid in soil: results from in situ soil column experiments. Chemosphere. 2010;79(4):401–7.
Levis TE, Wolfinger TF, Barta ML. The ecological effects of trichloroacetic acid in the environment. Environ Int. 2004;30:1119–50.
Hanson MR, Solomon KR. Haloacetic acids in the aquatic environment. Part II: ecological risk assessment. Environ Pollut. 2004;130:385–401.
Zeleňák V, Györyová K, Simon J. Thermal properties of zinc(II) chloroacetate and its complexes with nicotinamide and caffeine. J Therm Anal. 1996;46(2):573–9.
Ghule AV, Ghule K, Chen C-Y, Chen W-Y, Tzing S-H, Chang H, Ling Y-C. In situ thermos-TOF-SIMS study of thermal decomposition of zinc acetate dihydrate. J Mass Spectrom. 2004;39:1202–8.
Yun D, Li J, Yang X, Hu L, Wang Z, Liu Y, Wang C. Kinetic analysis on the non-isothermal dehydration by integral master-plots method and TG-FTIR study of zinc acetate dihydrate. J Anal Appl Pyrolysis. 2008;83:1–6.
McAdie HG. Crystal structure of µ4-Oxo-hexakis (µ-acetato) tetrazinc and thermal studies of its precursor, zinc acetate dihydrate. J Inorg Nucl Chem. 1966;28:2801–9.
Ghule AV, Lo B, Tzing S-H, Ghule K, Chang H, Ling YC. Simultaneous thermogravimetric analysis and in situ thermos-Raman spectroscopic investigation of thermal decomposition of zinc acetate dihydrate forming zinc oxide nanoparticles. Chem Phys Lett. 2003;381:262–70.
Mohamed MA, Halawy S, Ebrahim MM. Non-isothermal kinetic and thermodynamic study of the decomposition of lead acetate trihydrate. Thermochim Acta. 1994;236:249–62.
Harrison W, Trotter J. Crystal and molecular structure of cadmium diacetate dihydrate. J Chem Soc Dalton Trans. 1972;956–60.
Małecka B. Thermal decomposition of Cd(CH3COO)2·2H2O studied by a coupled TG–DTA–MS method. J Therm Anal Calorim. 2004;78:535–44.
Ивaнoв BB, Шyбин AA, Иpтюгo ЛA. The CdO powders synthesis by a thermal decomposition of unstable salts for arcing electro contact materials. J Sib Fed Univ Eng Technol. 2009;4:409–17.
Sun X, Yang J, Zhang W, Zhu X, Hu Y, Yang D, Yuan X, Yu W, Dong J, Wang H, Li L, Vasant Kumar R, Liang S. Lead acetate trihydrate precursor route to synthesize novel ultrafine lead oxide from spent lead acid battery postes. J Power Sources. 2014;269:565–76.
Szunyogovà E, Mudroňovà D, Györyovà K, Nemcovà R, Kovàřovà J, Piknovà-Findoràkovà L. The physicochemical and biological properties of zinc(II) complexes. J Therm Anal Calorim. 2007;2:355–61.
Györyovà K, Balek V. Thermal stability of new zinc acetate-based complex compounds. J Therm Anal. 1993;40:519–32.
Lin Chin-Cheng, Li Yuan-Yao. Synthesis of ZnO nanowires by thermal decomposition of zinc acetate dehydrate. Mater Chem Phys. 2009;113:334–7.
Powder Diffraction File, PDF-2. The International Centre for Diffraction Data (ICDD) 12 Campus Boulevard, Newton Square, PA, USA; 2004.
Geary WI. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev. 1971;7:81–122.
Spinner E. The vibration spectra of some substituted acetate ions. J Chem Soc 1966;4217.
Deacon GB, Philips RI. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev. 1980;33:227–50.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds J. New York: Wiley; 2009.
Manhas BS, Trikha AK. Relationships between the direction of shifts in the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Indian J Chem. 1982;59:315–9.
Brzyska W, Ożga W. Spectral, magnetic and thermal investigations of some d-electron element 3-methoxy-4-methylbenzoates. J Therm Anal Calorim. 2006;84:385–9.
Zeleňák V, Vargovà Z, Györyová K. Correlation of infrared spectra of zinc(II) carboxylates with their structures. Spectrochim Acta A. 2007;66:262–72.
Acknowledgements
We thank students M. Staniaszek and A. Stępień for participation part of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czylkowska, A., Raducka, A. & Mierczyński, P. Synthesis, thermal study and some properties of Zn(II), Cd(II) and Pb(II) compounds with mono-, di- and trichloroacetates. J Therm Anal Calorim 128, 937–946 (2017). https://doi.org/10.1007/s10973-016-5940-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5940-6