Abstract
Implementation of double cable models to simulate the behavior of myelinated peripheral nerve fibers requires defining a segmentation of the internode between successive nodes of Ranvier. The number of internodal segments is a model parameter that is not well agreed on, with values in the literature ranging from 1 to more than 500. Moreover, a lot of studies also lack a sensitivity study or a rationale behind the implementation used. In a model of a myelinated nerve fiber developed in our group, the segmentation scheme (i.e., the number of segments and their individual morphology) strongly influenced model outcomes such as action potential shape and velocity, stimulation threshold and absolute refractory period. In the present study these influences were investigated systematically in homogeneous neurons with different diameters. Uniformly segmented internodes were found to require several hundreds of segments (and associated computational power) to reach model outcomes differing by less than 1 % from the asymptotic value. In fact, in the majority of segmentation schemes the main determinant is not the number of segments, but the length λ of the internodal segments directly adjacent to the nodes of Ranvier. If λ is larger than approximately 10 μm, model outcomes for the tested fibers are almost independent of the total number of segments. Furthermore, λ can be optimized to enable models using just three segments per internode, to reach physiologically relevant model outcomes with limited computational resources. However, to study anatomical or physiological details of the internode itself, an appropriately detailed segmentation scheme is crucial.





Similar content being viewed by others
Notes
http://senselab.med.yale.edu/modeldb/showmodel.asp?model=3810&file=\mrgaxon\, accessed 1 March 2013.
References
Berthold, C. H., (1978). Morphology of normal peripheral axons. Physiology and Pathobiology of Axons, Ed. New York, Raven Press, 3–63.
Berthold, C. H., & Rydmark, M. (1983). Electrophysiology and morphology of myelinated nerve fibers. VI. Anatomy of the paranode-node-paranode region in the cat. Experientia, 39(9), 964–976.
Blight, A. R. (1985). Computer simulation of action potentials and afterpotentials in mammalian myelinated axons: the case for a lower resistance myelin sheath. Neuroscience, 15(1), 13–31.
Briaire, J. J., & Frijns, J. H. M. (2005). Unraveling the electrically evoked compound action potential. Hearing Research, 205(1), 143–156.
Cameron, T. (2004). Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. Journal of Neurosurgery: Spine, 100(3), 254–267.
Colombo, J., & Parkins, C. W. (1987). A model of electrical excitation of the mammalian auditory-nerve neuron. Hearing Research, 31(3), 287–311.
Dimitrov, A. G. (2005). Internodal sodium channels ensure active processes under myelin manifesting in depolarizing afterpotentials. Journal of Theoretical Biology, 235(4), 451–462.
Frijns, J. H. M., & Ten Kate, J. H. (1994). A model of myelinated nerve fibres for electrical prosthesis design. Medical and Biological Engineering and Computing, 32(4), 391–398.
Frijns, J. H. M., Briaire, J. J., de Laat, J. A. P. M., & Grote, J. J. (2002). Initial evaluation of the Clarion CII cochlear implant: speech perception and neural response imaging. Ear and Hearing, 23(3), 184–197.
Halter, J. A., & Clark, J. W. (1991). A distributed-parameter model of the myelinated nerve fiber. Journal of Theoretical Biology, 148(3), 345–382.
Hamani, C., Richter, E., Schwalb, J. M., & Lozano, A. M. (2005). Bilateral subthalamic nucleus stimulation for Parkinson’s disease: a systematic review of the clinical literature. Neurosurgery, 56(6), 1313–1324.
Hines, M. L., & Carnevale, N. T. (1997). The NEURON simulation environment. Neural Computation, 9(6), 1179–1209.
Jezernik, S., Craggs, M., Grill, W., Creasey, G., & Rijkhoff, N. (2002). Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurological Research, 24(5), 413–430.
McIntyre, C. C., Richardson, A. G., & Grill, W. M. (2002). Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. Journal of Neurophysiology, 87(2), 995–1006.
McNeal, D. R. (1976). Analysis of a model for excitation of myelinated nerve. Biomedical Engineering, IEEE Transactions, 23(4), 329–337.
Rattay, F., Lutter, P., & Felix, H. (2001). A model of the electrically excited human cochlear neuron: I. Contribution of neural substructures to the generation and propagation of spikes. Hearing Research, 153(1), 43–63.
Schwarz, J. R., Reid, G., & Bostock, H. (1995). Action potentials and membrane currents in the human node of Ranvier. Pflügers Archiv European Journal of Physiology, 430(2), 283–292.
Smit, J. E., Hanekom, T., Van Wieringen, A., Wouters, J., & Hanekom, J. J. (2010). Threshold predictions of different pulse shapes using a human auditory nerve fibre model containing persistent sodium and slow potassium currents. Hearing Research, 269(1), 12–22.
Stephanova, D. I., & Bostock, H. (1995). A distributed-parameter model of the myelinated human motor nerve fibre: temporal and spatial distributions of action potentials and ionic currents. Biological Cybernetics, 73(3), 275–280.
Waxman, S. G., & Ritchie, J. M. (1985). Organization of ion channels in the myelinated nerve fiber. Science, 228(4707), 1502–1507.
Wesselink, W. A., Holsheimer, J., & Boom, H. B. (1999). A model of the electrical behaviour of myelinated sensory nerve fibres based on human data. Medical and Biological Engineering and Computing, 37(2), 228–235.
Young, R. G., Castelfranco, A. M., & Hartline, D. K. (2013). The “Lillie Transition”: models of the onset of saltatory conduction in myelinating axons. Journal of Computational Neuroscience, 1–14.
Acknowledgments
This study was supported financially by Stichting Het Heinsius-Houbolt Fonds.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Frances K. Skinner
Appendix
Appendix
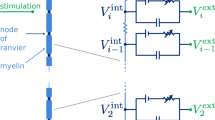
In order to be able to apply a single formula for the transneural potential in different neural compartments (i.e., in both nodes of Ranvier and internodal segments), all neural compartments were labeled by a single index n. This way, if for example 5 internodal segments were modeled per internode, the first node of Ranvier would be labeled n = 1, the 5 consecutive internodal segments n = 2, 3,...6, the second node of Ranvier n = 7, etc. Application of Kirchhoff’s law in a neural compartment (e.g., the point labeled ‘n’ in Figure 1) yields the following equation for the transneural potential V n :
\( \begin{array}{l}\frac{d{V}_n}{ dt}=\frac{d{\displaystyle {V}_n^{\prime }}}{ dt}+\\ {}\frac{G_{n-1}^a}{C_n^{my}}\left[\varDelta {V}_{n-1}+\varDelta {V}_{n-1}^e\right]+\frac{G_n^a}{C_n^{my}}\left[\varDelta {V}_{n+1}+\varDelta {V}_{n+1}^e\right]+\\ {}\frac{G_{n-1}^p}{C_n^{my}}\left[\varDelta {V}_{n-1}-\varDelta {\displaystyle {V}_{n-1}^{\prime }}+\varDelta {V}_{n-1}^e\right]+\\ {}\frac{G_n^p}{C_n^{my}}\left[\varDelta {V}_{n+1}-\varDelta {\displaystyle {V}_{n+1}^{\prime }}+\varDelta {V}_{n+1}^e\right]-\frac{G_n^{my}}{C_n^{my}}\left[{V}_n-{\displaystyle {V}_n^{\prime }}\right]\end{array} \)A.1
with the transmembrane voltage V ′ n defined by
\( \begin{array}{l}\frac{d{\displaystyle {V}_n^{\prime }}}{ dt}=-\frac{G_n^L}{C_n^m}\left[{\displaystyle {V}_n^{\prime }}-{V}^L\right]-\frac{1}{C_n^m}{I}_n^{act}\left({\displaystyle {V}_n^{\prime }}\right)+\\ {}\frac{G_{n-1}^a}{C_n^m}\left[\varDelta {V}_{n-1}+\varDelta {V}_{n-1}^e\right]+\frac{G_n^a}{C_n^m}\left[\varDelta {V}_{n+1}+\varDelta {V}_{n+1}^e\right]\end{array} \)A.2
In equations A.1 and A.2, G a n , G p n ,
G my n and G L n are the axonal conductance, the conductance of the peri-axonal space and the conductance of the myelin layer at node n and the passive leakage conductance.
C a n , C p n , and C my n resemble the corresponding capacitances. G my n and C my n are calculated by:
\( {G}_n^{my}=2\pi \left[{R}_n+{\delta}^p+\frac{N_n{\delta}^{my}}{2}\right]{L}_n\frac{g_{my}}{N_n} \)A.3
\( {C}_n^{my}=2\pi \left[{R}_n+{\delta}^p+\frac{N_n{\delta}^{my}}{2}\right]{L}_n\frac{c_{my}}{N_n} \)A.4
in which R n is the axonal radius, δ p is the thickness of the periaxonal layer, N n is the number of myelin layers, δ my is the myelin thickness, L n is the axonal length of the nth neural compartment and c my and g my are the capacitance and conductance of the myelin per unit area. I act n is the sum of currents arising from the ion flow through the nodal voltage gated ion channels, the equations for which were taken from Schwarz et al. (1995), from which also the electrical and kinetics parameters were used, together with the correction by Wesselink et al. (1999). ΔV n − 1 = V n − 1 − V n , ΔV n + 1 = V n + 1 − V n and V e n is the extracellular potential. The latter voltages all are relative to a resting potential E r , which was calculated by the Goldman equation. As already mentioned in the text, the equations were solved using a backward Euler integration algorithm with a standard step size of 0.001 μs, as it turned out that all results were stable for time step sizes of 0.001 μs and smaller.
Rights and permissions
About this article
Cite this article
Dekker, D.M.T., Briaire, J.J. & Frijns, J.H.M. The impact of internodal segmentation in biophysical nerve fiber models. J Comput Neurosci 37, 307–315 (2014). https://doi.org/10.1007/s10827-014-0503-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-014-0503-y