Abstract
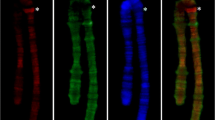
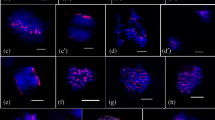
Eukaryotic chromosomes are organized into two large and distinct domains, euchromatin and heterochromatin, which are cytologically characterized by different degrees of chromatin compaction during interphase/prophase and by post-synthesis modifications of histones and DNA methylation. Typically, heterochromatin remains condensed during the entire cell cycle whereas euchromatin is decondensed at interphase. However, a fraction of the euchromatin can also remain condensed during interphase and appears as early condensing prophase chromatin. 5S and 45S rDNA sites and telomere DNA were used to characterize these regions in metaphase and interphase nuclei. We investigated the chromosomal distribution of modified histones and methylated DNA in the early and late condensing prophase chromatin of two species with clear differentiation between these domains. Both species, Costus spiralis and Eleutherine bulbosa, additionally have a small amount of classical heterochromatin detected by CMA/DAPI staining. The distribution of H4 acetylated at lysine 5 (H4K5ac), H3 phosphorylated at serine 10 (H3S10ph), H3 dimethylated at lysine 4 or 9 (H3K4me2, H3K9me2), and 5-methylcytosine was compared in metaphase, prophase, and interphase cells by immunostaining with specific antibodies. In both species, the late condensing prophase chromatin was highly enriched in H4K5ac and H3K4me2 whereas the early condensing chromatin was very poor in these marks. H3K9me2 was apparently uniformly distributed along the chromosomes whereas the early condensing chromatin was slightly enriched in 5-methylcytosine. Signals of H3S10ph were restricted to the pericentromeric region of all chromosomes. Notably, none of these marks distinguished classical heterochromatin from the early condensing euchromatin. It is suggested that the early condensing chromatin is an intermediate type between classical heterochromatin and euchromatin.





Similar content being viewed by others
References
Barlow PN (1977) Determinants of nuclear chromatin structure in angiosperms. Nat Bot Biol Veg 18:193–206
Brasileiro-Vidal AC, Melo-Oliveira MB, Carvalheira GMG, Guerra M (2009) Different chromatin fractions of tomato (Solanum lycopersicum L.) and related species. Micron 40:851–859
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304
Chang SB, Yang TJ, Datema E et al (2008) FISH mapping and molecular organization of the major repetitive sequences of tomato. Chromosom Res 16:919–933
Cremonini R, Ruffini Castiglione M, Grif VG et al (2003) Chromosome banding and DNA methylation patterns, chromatin organization and nuclear DNA content in Zingeria biebersteiniana. Biol Plantarum 46:543–550
Dantas LG, Guerra M (2010) Chromatin differentiation between Theobroma cacao L. and T. grandiflorum. Genet Mol Biol 33:94–98
Delay C (1949) Recherches sur la structure des noyaux quiescents chez les phanérogames. Rev Cytol Cytophysiol Veg 10:103–228
Dhar MK, Fuchs J, Houben A (2009) Distribution of eu- and heterochromatin in Plantago ovata. Cytogenet Genom Res 125:235–240
Fransz P, Hans de Jong J, Lysak M, Ruffini Castiglione M, Schubert I (2002) Interphase chromosomes in Arabidopsis are organized as well defined chromo centers from which euchromatin loops emanate. Proc Natl Acad Sci USA 99:14584–14589
Fransz P, ten Hoopen R, Tessadori F (2006) Composition and formation of heterochromatin in Arabidopsis thaliana. Chromosom Res 14:71–82
Fuchs J, Demidov D, Houben A, Schubert I (2006) Chromosomal histone modification patterns–from conservation to diversity. Trends Plant Sci 11:199–208
Fuks F (2005) DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev 15:1–6
Guerra M (1987) Cytogenetics of Rutaceae IV. Structure and systematic significance of interphase nuclei. Cytologia 52:213–222
Guerra M (1988a) Characterization of different types of condensed chromatin in Costus (Zingiberaceae). Plant Syst Evol 158:107–115
Guerra M (1988b) Mitotic and meiotic analysis of a pericentric inversion associated with a tandem duplication in Eleutherine bulbosa. Chromosoma 97:80–87
Guerra M, Brasileiro-Vidal AC, Arana P, Puertas MJ (2006) Mitotic microtubule development and histone H3 phosphorylation in the holocentric chromosomes of Rhynchospora tenuis (Cyperaceae). Genetica 126:33–41
Heitz E (1928) Das Heterochromatin der Moose. I. Jahrb Wiss Bot 69:762–818
Hendzel MJ, Wei Y, Mancini MA et al (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360
Holmquist GP, Ashley T (2006) Chromosome organization and chromatin modification: influence on genome function and evolution. Cytogenet Genom Res 114:96–125
Houben A, Demidov D, Gernand D, Meister A, Leach CR, Schubert I (2003) Methylation of histone H3 in euchromatin of plant chromosomes depends on basic nuclear DNA content. Plant J 33:967–973
Houben A, Demidov D, Caperta AD, Karimi R, Agueci F, Vlasenko L (2007) Phosphorylation of histone H3 in plants—a dynamic affair. Biochim Biophys Acta 1769:308–315
Jackson JP, Johnson L, Jasencakova Z et al (2004) Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112:308–315
Jin W, Lamb JC, Zhang W, Kolano B, Birchler JA, Jiang J (2008) Histone modifications associated with both A and B chromosomes of maize. Chromosom Res 16:1203–1214
Kouzarides T (2007) Review chromatin modifications and their function. Cell 128:693–705
Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11:204–220
Manzanero S, Rutten T, Kotseruba V, Houben A (2002) Alterations in the distribution of histone H3 phosphorylation in mitotic plant chromosomes in response to cold treatment and the protein phosphatase inhibitor cantharidin. Chromosom Res 10:467–476
Miller OJ, Schnedl W, Allen J, Erlanger BF (1974) 5-Methylcytosine localised in mammalian constitutive heterochromatin. Nature 251:636–637
Nagl W (1979) Condensed interphase chromatin in plant and animal cell nuclei: fundamental of genetic information. Nature 447:951–958
Pedrosa A, Sandal N, Stougaard J, Schweizer D, Bachmair A (2002) Chromosomal map of the model legume Lotus japonicus. Genetics 161:1661–1672
Ribeiro T, Viegas W, Morais-Cecílio L (2009) Epigenetic marks in the mature pollen of Quercus suber L. (Fagaceae). Sex Plant Reprod 22:1–7
Richards E, Ausubel FM (1988) Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell 53:127–136
Ruffini Castiglione M, Giraldi E, Frediani M (1995) The DNA methylation pattern of Allium cepa metaphase chromosomes. Biol Zentralbl 114:57–66
Schotta G, Lachner M, Sarma K et al (2004) A silencing pathway to induce H3–K9 and H4–K20 trimethylation at constitutive heterochromatin. Gene Dev 18:1251–1262
Vyskot B, Siroky J, Hladilova R, Belyaev ND, Turner BM (1999) Euchromatic domains in plant chromosomes as revealed by H4 histone acetylation and early DNA replication. Genome 42:343–350
Wanzenböck EM, Schöfer C, Schweizer D, Bachmair A (1997) Ribosomal transcription units integrated via T-DNA trans-formation associate with the nucleolus and do not require up-stream repeat sequences for activity in Arabidopsis thaliana. Plant J 11:1007–1016
Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD (1998) Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA 95:7480–7484
Wolf KW, Turner BM (1996) The pattern of histone H4 acetylation on the X chromosome during spermatogenesis of the desert locust Schistocerca gregaria. Genome 39:854–865
Acknowledgments
The authors wish to thank the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for financial support, and Cibele Caio, Magdalena Vaio, and Paola Gaiero for corrections to the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feitoza, L., Guerra, M. Different types of plant chromatin associated with modified histones H3 and H4 and methylated DNA. Genetica 139, 305–314 (2011). https://doi.org/10.1007/s10709-011-9550-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-011-9550-8