Abstract
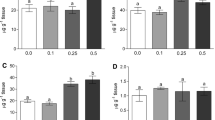
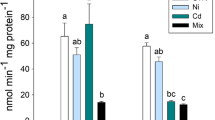
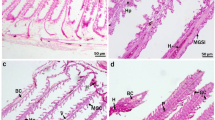
Contamination of aquatic ecosystems by metals causes various biochemical changes in aquatic organisms, and fish are recognized as indicators of environmental quality. Silver catfish were exposed to six concentrations of zinc (Zn): 1.0, 2.5, 5.0, 7.5, 10.0 and 12.5 mg/L for 96 h to determine the mean lethal concentration (LC50). The value obtained was 8.07 mg/L. In a second experiment, fish were exposed to concentrations of 1.0 or 5.0 mg/L Zn and a control for 96 h. Afterward, the tissues were collected for biochemical analysis. Lipid peroxidation, as indicated by thiobarbituric acid-reactive substance (TBARS), decreased in the liver and brain for all Zn concentrations tested, while in the gills TBARS levels increased at 1.0 mg/L and declined at 5.0 mg/L. Zn increased protein carbonyls in the muscle of silver catfish and decreased it in the other tissues. The enzyme superoxide dismutase increased in both exposed groups. However, catalase did not change. Glutathione S-transferase decreased in the liver and increased in the gills (1.0 mg/L), muscle (5.0 mg/L) and brain (1.0 and 5.0 mg/L). Nonprotein thiols changed only in brain and muscle tissue. Zn exposure inhibited acetylcholinesterase (AChE) activity in the brain at both concentrations tested, but did not change it in muscle. Exposure to Zn inhibited the activity of Na+/K+-ATPase in the gills and intestine at both concentrations tested. Our results demonstrate that Zn alters biochemical parameters in silver catfish and that some parameters such as AChE and Na+/K+-ATPase could be considered as early biomarkers of waterborne Zn toxicity.




Similar content being viewed by others
References
Alam M, Frankel T (2006) Gill ATPase of silver perch, Bidyanus bidyanus (Mitchell), and golden perch, Macquaria ambigua (Richardson): effects of environmental salt and ammonia. Aquaculture 251:118–133
Almeida JA, Diniz YS, Marquez SFG, Faine LA, Ribas BO, Burneiko RC, Novelli ELB (2002) The use of the oxidative stress as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ Int 27:673–679
Atli G, Canli M (2007) Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp Biochem Physiol C: Toxicol Pharmacol 145:282–287
Baysoy E, Atli G, Canli M (2013) The effects of salinity and salinity + metal (chromium and lead) exposure on ATPase activity in the gill and intestine of tilapia Oreochromis niloticus. Arch Environ Contam Toxicol 64:291–300
Bianchini A, Castilho PC (1999) Effect of zinc exposure on oxygen consumption and gill Na+K+-ATPase of the estuarine crab Chasmagnathus granulate Dana, 1951 (Decapoda–Grapsidae). Bull Environ Contam Toxicol 62:63–69
Bradford MMA (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–309
Campana O, Sarasquete C, Blasco J (2003) Effecton on ALA-D activity, metallothionein levels, and lipid peroxidation in blood, kidney, and liver of the toadfish Halobatrachus didactylus. Ecotoxicol Environ Saf 55:116–125
Canli M, Stagg RM (1996) The effects of in vivo exposure to cadmium, copper, and zinc on the activities of gill ATPases in the Norway lobster Nephrops norvegicus. Arch Environ Contam Toxicol 31:491–501
CONAMA (Conselho Nacional do Meio Ambiente) (2005) Resolução CONAMA nº357 de 17/03/05. http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=459. Accessed 22 Jan 2015
Dautremepuits C, Paris-Palacios S, Betoulle S, Vernet G (2004) Modulation in hepatic and head kidney parameters of carp (Cyprinus carpio L.) induced by copper and chitosan. Comp Biochem Physiol C: Toxicol Pharmacol 137:325–333
Deneke SM, Fanburg BL (1989) Regulation of cellular glutathione. Am J Physiol 257:163–173
Ebrahimpour M, Alipour H, Rakhshah S (2010) Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicol Ind Health 26:361–365
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem 82:70–77
Ellman GL, Courtney KD, Andres V Jr (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environment pollution in African cat fish (Clarias gariepinus) from Nigeria Ogum River. Int J Environ Res Public Health 4:158–165
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Galloway TS, Millward N, Browne MA, Depledge MH (2002) Rapid assessment of organophosphorus/carbamate exposure in the bivalve mollusk Mytilus edulis using combined esterase activities as biomarkers. Aquat Toxicol 61:169–180
Gioda CR, Lissner LA, Pretto A, da Rocha JB, Schetinger MR, Neto JR, Morsch VM, Loro VL (2007) Exposure to sublethal concentrations of Zn (II) and Cu (II) changes biochemical parameters in Leporinus obtusidens. Chemosphere 69:170–175
Grosell M, Wood CM, Walsh PJ (2003) Copper homeostasis and toxicity in the elasmobranch Raja erinacea and the teleost Myoxocephalus octodecemspinosus during exposure to elevated waterborne copper. Comp Biochem Physiol C 135:179–190
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge J (2007) Free radicals in biology and medicine. Oxford University Press, New York
Heath AG (1987) Water pollution and fish physiology. CRC Press, Florida, p 245
Hermes-Lima M (2004) Principles of metabolic control. In: Storey K (ed) Functional metabolism: regulation and adaptation. Wiley
Hershfinkel M, Silverman WF, Sekler I (2007) The zinc sensing receptor, a link between zinc and cell signaling. Mol Med 13:331–336
Huang EP (1997) Metal ions and synaptic transmission: think zinc. Proc Natl Acad Sci USA 94:13386–13387
Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330
Loro VL, Jorge MB, Silva KR, Wood CM (2012) Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in euryhaline teleost Fundulus heteroclitus: protective effects of salinity. Aquat Toxicol 110–111:187–193
Loro VL, Nogueira L, Nadella SR, Wood CM (2014) Zinc bioaccumulation and ionoregulatory impacts in Fundulus heteroclitus exposed to sublethal waterborne zinc at different salinities. Comp Biochem Physiol Part C 166:96–104
Lushchak V (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
MacDonald RS (2000) The role of zinc in growth and cell proliferation. J Nutr 130:1500s–1508s
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
McGeer JC, Szebedinszky C, McDonald DG, Wood CM (2000) Effect of chronic sublethal exposure to waterborne Cu, Cd, or Zn in rainbow trout 1: ionoregulatory disturbance and metabolic costs. Aquat Toxicol 50(3):231–243
Meyer JN, Smith JD, Winston GW, Di Giulio RT (2003) Antioxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model pro-oxidants: short-term and heritable responses. Aquat Toxicol 65:377–395
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Nelson DP, Kiesow LA (1972) Enthalphy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solution in the UV). Anal Biochem 49:474–478
Oteiza PI, Mackenzie GG (2005) Zinc, oxidant-triggered cell signaling, and human health. Mol Aspects Med 26:245–255
Pandey S, Parvez S, Ansari RA, Ali M, Kaur M, Hayat F, Ahmad F, Raisuddin S (2008) Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chem Biol Interact 174:183–192
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145:321–329
Santos DCM, Matta SLP, Oliveira JA, Santos JAD (2012) Histological alterations in gills of Astyanax aff. bimaculatus caused by acute exposition to zinc. Exp Toxicol Pathol 64:861–866
Shaw BJ, Al-Bairuty G, Handy RD (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): physiology and accumulation. Aquat Toxicol 116–117:90–101
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29(12):1715–1733
Tamir HA, Sharir H, Schwartz B, Hershfinkel M (2004) Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J Biol Chem 279:51804–51816
Vallee BL (1988) Zinc: biochemistry, physiology, toxicology and clinical pathology. BioFactors 1:31–36
Viarengo A, Burlando B, Ceratto N, Panfoli I (2000) Antioxidant role of metallothioneins: a comparative overview. Cell Mol Biol 46:407–417
Winston GW (1991) Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C 100:173–176
Xu YJ, Liu XZ, Ma AJ (2004) Current research on toxicity effect and molecular mechanism of heavy metals on fish. Mar Sci 28:67–70
Yan LJ, Traber MG, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351
Acknowledgments
We would like to thank the Federal University of Santa Maria (UFSM) for the support and facilities and the financial support and fellowships from the Brazilian agencies CAPES (Coordination for the Improvement of Higher Education Personnel) and CNPq (National Council for Scientific and Technological Development).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leitemperger, J., Menezes, C., Santi, A. et al. Early biochemical biomarkers for zinc in silver catfish (Rhamdia quelen) after acute exposure. Fish Physiol Biochem 42, 1005–1014 (2016). https://doi.org/10.1007/s10695-015-0192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0192-0