Abstract
Nsa cytoplasmic male sterility (CMS) is a novel Brassica napus male sterility system derived from Sinapis arvensis cytoplasm. Nsa CMS results in defective pollen production due to S. arvensis mitochondrial gene failure/incompatibility in the anthers, requiring nuclear genes to restore fertility. From ultramicroscopic observation of anther sections, we concluded that the induction of sterility begins at the pollen (microspore) mother cell stage. Most pollen mother cells do not undergo the first meiotic division and dissociate before the tetrad stage. At the tetrad stage, abortion was observed for all uninucleate pollen. Dysfunction of mitchondrial gene(s) leads to cell vacuolization in the anther tapetum and middle layer cells. Early programmed cell death (PCD) of the tapetum and anther middle layer cells is the main reason for pollen mother cell abortion. Due to this early pollen abortion, the sterility of Nsa CMS is stable and complete. Southern blotting of DNA from the Nsa male-sterile line, its maintainer and restorer lines, as well as the two parental lines (B. napus cv. Zhongshuang 4 and S. arvensis var. Yeyou 18) involved in the somatic hybridization, suggested that the cytoplasm of the Nsa CMS line was from S. arvensis and that of the restorer line was a rearrangement of both parental lines. Nsa CMS shows great potential for hybrid seed production in rapeseed. Our results provide clues to identify novel male-sterility (S) and restorer (R) genes as well as elucidate the mechanism underlying interactions between the S and R genes.
Similar content being viewed by others
Introduction
Hybrid breeding, whereby two inbred parent lines are crossed to generate first-generation hybrids with increased yield due to heterosis, is an important process in many crops, including the economically significant oilseed crop Brassica napus (rapeseed or canola). Unfortunately, hybrid breeding requires production of new hybrid seed in each generation from crosses between the two parent lines, as self-pollination of hybrids results in loss of heterosis and hence seed yield. In preferentially self-pollinating crops such as B. napus, high-throughput production of hybrid seed relies on the use of male sterility systems. One parent line is induced to be male sterile, such that it is incapable of self-pollination, and the other desired parent line is planted next to it with a “restorer” gene that allows pollen from this parent to fertilize the adjacent male-sterile line to produce viable seed. Cytoplasmic male sterility (CMS) is the main type of male sterility used for hybrid breeding in rapeseed (Virmani and Edwards 1983). To date, only two CMS systems with stable male sterility and a high degree of fertility restoration have been widely used in rapeseed hybrid production: Ogura and Polima (Zhou and Fu 2007). The development of commercial hybrid rapeseed remains of great interest, and a key to success will be improvement of hybrid production efficiency, for which better sources of male sterile cytoplasm are a critical requirement.
CMS is a maternally inherited trait in higher plants that prevents the production of functional pollen. CMS is a universal phenomenon that has been described in over 150 plant species and is thought to derive from abnormal recombination events between the mitochondrial genome and the nuclear genome in most species (Budar and Pelletier 2001). This recombination between the mitochondrial and nuclear genomes may result in abnormal transcripts, which may underlie the CMS character (Kadowaki et al. 1990; Kini et al. 1994). The abnormal recombination generates new open reading frames (ORFs), which are usually co-transcribed with functional mitochondrial genes, thus leading to mitochondrial dysfunction. In most CMS plants, anthers develop normally but microspore genesis is arrested during or soon after meiosis (Kaul 1988). Generally, the stagnancy of microspore genesis is thought to be the result of the tapetum undergoing cell degradation during meiosis, which does not occur naturally in fertile plants. In CMS lines of sunflower, it has been suggested that a deregulated programmed cell death (PCD) program causes the death of tapetum cells and microspore abortion (Balk and Leaver 2001). Alloplasmy, whereby the cytoplasm is from a different species than the nuclear genome, often leads to disharmony between the mitochondrial genome and the nuclear genome (Bereterbide et al. 2002).
Cytological studies of CMS have revealed various causes of infertility and abortion of male gametes. In nap CMS, a type of CMS found in natural B. napus, infertility is due to adhesion among anthers and delayed pollen development (Shiga and Baba 1973). In Ogura CMS, anther abortion begins at the uninucleate microspore stage, with inhibited release of microspores from the tetrads (Pelletier et al. 1983; Gonzalez-Melendi et al. 2008). In Polima CMS, anther abortion is initiated during microspore cell differentiation due to failed formation of the pollen sac (Fu 1981; Yu and Fu 1990). CMS 212A anther development was investigated using paraffin-sectioning microscopy, and 212A anthers were found to be retarded at an early cell stage (Liu et al. 2005): anthers were composed of vascular elements, without pollen mother cell formation and differentiation within the pollen sac. Wei et al. (2005) conducted microscopic and ultra-structural observations on Nca CMS lines derived from interspecific hybridization between B. napus and B. carinata. Male sterility in the Nca CMS lines was initiated at the late uninucleate pollen stage: pollen mother cells progressed through meiosis and released microspores from the tetrads, and microspores formed early pollen walls, but later in the uninucleate pollen development stage the cytoplasm and nucleus degraded, leaving only small vacuoles inside the pollen grain. Anther developmental delay occurred because of the complete degradation of the association between the tapetum and pollen mother cells with severe vacuolization.
Nsa CMS is a novel type of alloplasmic male sterility system in B. napus obtained by somatic hybridization between B. napus and S. arvensis (Hu et al. 2002). The male sterility of Nsa CMS is complete and stable. The Nsa CMS system including male-sterile and restorer lines also possesses valuable traits such as tolerance to stem rot (Sclerotinia sclerotiorum) and drought as well as a low incidence of pod shattering (Wei et al. 2010). Application of the Nsa CMS system in hybrid seed production not only increases the genetic diversity of the hybrid rapeseed cytoplasm, but also potentially improves hybrid seed purity because of the high stability of the male sterility under different environmental conditions. We aimed to further investigate the Nsa CMS system and identify the stage of pollen developmental abortion. First, we conducted cytological observations on anthers of Nsa CMS and their maintainer lines in order to identify the causal cytological mechanism for pollen abortion. Second, we studied the origin of the mitochondrial genomes of the male sterile and restorer lines as well as their parents through Southern blot analyses. The possible roles of mitochondrial genetics in the male sterile, maintainer and restorer lines of the Nsa CMS system and the cause of sterility in this system are discussed.
Materials and methods
Plant material
The experimental material consisted of B. napus “Zhongshuang 4” and S. arvensis “Yeyou 18.” Nsa CMS male sterile lines were produced by somatic hybridization between B. napus cv. “Zhongshuang 4” and S. arvensis cv. “Yeyou 18,” followed by successive back-crosses of sterile somatic hybrids with B. napus as the male parent (Hu et al. 2002). Nsa CMS restorer lines were obtained by pedigree selection of fertile somatic hybrids (Wei et al. 2010).
Light microscopy and ultrathin sectioning
One anther of a flower bud was observed under an optical microscope to determine the anther developmental stage, and the remaining anthers from the same flower were fixed for further observation. The samples were fixed in FAA fixative solution containing 37 % formaldehyde, 70 % ethanol and 100 % acetic acid (18:1:1). Anthers were collected and dehydrated through an ethanol series of 70, 85, 95 and 100 % (v/v). The anthers were pre-fixed in a solution of 2.5 % glutaraldehyde adjusted to pH 7.4 with phosphate buffer, fixed in 1 % OsO4 in the same buffer, and then dehydrated and embedded in Spurr resin. Ultrathin sections (approximately 60–70 nm) obtained with a Leica EM UC6 ultramicrotome were stained with 2 % uranyl acetate and 1 % aluminum citrate for 30 min in the dark. The observation and recording of images were carried out with a Hitachi (Japan) H-7650 transmission electron microscope at 80 kV.
Southern blots
The plants used for mitochondrial DNA preparations were from the Nsa CMS, restorer, maintainer and two parental lines from the somatic hybridization combination. Mitochondria were prepared from seedlings growing in the dark at 30 °C using differential centrifugation (Wang et al. 2008); DNA was recovered from lysed mitochondria following phenol/chloroform extractions and ethanol precipitation as described previously (Uzunova et al. 1995). DNA was digested with the restriction endonucleases EcoRI, separated by size using electrophoresis (1 % agarose gel in 1 × TAE buffer) and then transferred to a Hybond™-N+ membrane (Invitrogen Biotech). The DNA blot was hybridized with 324 bp of atp9 sequence labeled with digoxigenin (Roche, USA) by PCR using the primers atp9f (5′cat gtc aat gct atg tcg atc3′) and atp9r (5′ tca gta atg cat cgg atg agt 3′). The Dig-labeled probe sequence amplifies a highly conserved mitochondrial gene sequence in Brassica species.
Results
Microscopy
The flower morphology of the Nsa sterile and fertile restorer lines is shown in Fig. 1. Petals of the Nsa CMS line were similar in shape and color to normal flowers, but had wrinkled petals (Fig. 1a, b). Compared to fertile flowers (Fig. 1c), the sterile flowers displayed normal pistil development, but contained completely degraded stamens with aborted microspores (Fig. 1d).
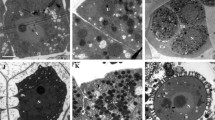
Microscopy revealed that the pollen mother cells were deformed, with only residual cells from the on-anther pollen mother cell development stage (Fig. 2f). Ultra-thin microscopy sectioning showed sterile pollen mother cells of various sizes during meiosis: most have begun to abort, and there is clear vacuolization in some pollen mother cells (Fig. 2f and g, black arrows) and even complete vacuolization in others (white arrows in Fig. 2f and g). Cytoplasmic degeneration was easy to observe, with breakdown of plastids and mitochondria at this stage (Fig. 2f and g inside the P and M). The middle layer of tapetum cells completely degraded (Fig. 2F in the ML), and only empty cell walls remained. By the tetrad stage, the tapetum structure was extremely disordered, and the callose layer had disintegrated. Tetrads only contained cavities rather than developed nuclei (Fig. 2h). Due to early tapetum degradation, microspores may have failed to obtain enough nutrients for development.
Transmission electron-microscopic observation of the anther development of Nsa CMS (f–j) in comparison to fertile Brassica napus (a–e). Black arrows indicate partial vacuolization, and white arrows indicate complete vacuolization in pollen mother cells. a The nucleolus of a pollen mother cell during meiosis at ×8000 magnification; b cross section of pollen mother cell during meiosis at ×3000 magnification; c cross section of pollen mother cell at the tetrad stage at ×3000 magnification; d cross section of pollen mother cell at the mononucleus stage at ×3000 magnification; e the middle layer of the anther at the mononucleus stage at ×5000 magnification. f Anther and pollen mother cell during meiosis at ×5000 magnification; g pollen mother cell during meiosis at ×4000 magnification; h pollen mother cell at the tetrad stage at ×3000 magnification; i tapetum at the mononucleus stage at ×2500 magnification; j pollen mother cell that was aborted completely at the mononucleus stage at ×3000 magnification
In the fertile anthers, pollens with regular size and shape were observed, with the three pores clearly visible (Fig. 2f), while CMS pollen was highly variable in size and irregularly shaped (Fig. 2i). The pollen grains of CMS anthers were surrounded by released degradation products (Fig. 2j), leaving only empty pollen walls. At this stage, single-nucleus anther tapetum was visible in the fertile anthers, but in the sterile anthers the tapetum was completely disintegrated.
Mitochondrial DNA probes
Mitochondrial DNA polymorphisms were detected using a mitochondrial DNA probe atp9 (Fig. 3). Nsa CMS showed two main bands (2000 and 8000 bp), matching the S. arvensis parent. A different banding pattern was found in the maintainer line and in the B. napus parent, with one band of 2500 bp. The band polymorphism of the restorer line (one band at 1500 bp and one at 3000 bp) was different from both the sterile and maintainer lines. The atp9 probe in combination with EcoRI restriction endonucleases allowed us to distinguish Nsa CMS cytoplasm from the maintainer and restorer lines. These results indicate that the mitochondrial genome of Nsa CMS might be derived from the S. arvensis parent rather than the B. napus parent, and rearrangements in the mitochondrial genome of the restorer line might have occurred.
Discussion
The Nsa CMS system appears to result from a disruption of anther development at a very early stage of pollen mother cell development. In contrast, the anther development of pol CMS is inhibited at the polarization stage of the microspore, aborting pollen sac formation (Yu and Fu 1990). The anther development of ogu CMS is inhibited at the tetrad to uninucleate pollen formation stage, with pollen autolysis at the vacuolated microspore stage (Gonzalez-Melendi et al. 2008). Developmental disruption of pol and ogu CMS systems occurs at a later stage than the Nsa CMS system, as pol and ogu CMS systems form relatively normal-looking anthers, but fail to develop normal microspore cells. This result indicates that Nsa CMS is unique and different from the other CMS systems in rapeseed. Through molecular identification and tests of the restorer and maintainer relationship, Cheng et al. (2008) also showed that the Nsa CMS cytoplasm is completely different from those of Ogu and Pol.
Pollen development is intimately linked to anther development (Scott et al. 2004). Abortion of microspores is often caused by an abnormal tapetum, which fails to transmit enough nutrients for microspore development. In addition, the tapetum cells produce enzymes that induce release of microspores from the tetrad after meiosis. A dynamic “biological dialogue” exists between the tapetum and pollen mother cells, and blocking of this “dialogue” at any point can initiate pollen abortion.
In Nsa CMS anthers, loss of normal function and disintegration of the tapetum were observed at the pollen mother cell stage. Pollen mother cells subsequently failed to obtain enough nutrients during the meiosis period, resulting in consumption of their own cytoplasm and causing large intracellular vacuoles and related phenomena. In addition, the middle tapetum layer was also completely dissociated at the pollen mother cell stage. Early degradation of the middle tapetum layer may indirectly affect tapetum degradation. However, previous studies have generally shown that abortion is only induced from abnormal tapetum.
Restriction fragment length polymorphism (RFLP) marker analysis has been successfully applied to identify interspecific and intergeneric somatic hybrids in Brassicaceae. Using atp9 and coxII as probes, Hu et al. (2002) identified somatic hybrids in early generation Brassica hybrid material. The atp9 hybridization patterns in their study showed that the four male sterile hybrids tested had the same hybridization pattern as S. arvensis, whereas two fertile plants appeared different, with one the same as S. arvensis and the other the same as B. napus. Nsa CMS and restorer lines in this study were derived from the offspring of the sterile and fertile somatic hybrid plants (Hu et al. 2002), respectively, and thus possess similar RFLP patterns when probed with atp9. As only one probe-enzyme combination was used for mitochondrial genome classification of Nsa CMS, it may not be sufficient to conclude that the mitochondrial composition of Nsa CMS was the same as that of S. arvensis, but it is clear that the mitochondrial genome of Nsa CMS is different from that of B. napus. To clearly characterize the cytoplasm source and composition of Nsa CMS, experimental evidence with more probe-enzyme combinations is needed.
It is interesting that the restorer line possesses an RFLP banding pattern different from those of the Nsa CMS line and their parental lines. Since no restorer line was identified from ordinary B. napus lines (Hu et al. 2003), and fertility restoration could only be fulfilled by lines derived from fertile somatic hybrids (Wei et al. 2010), it is likely that both genes responsible for cytoplasmic sterility and fertility restoration were derived from the S. arvensis parent. As the restorer line was developed by pedigree selection from a fertile cybrid, and the CMS line was derived from a sterile cybrid, it is reasonable that the CMS and restorer lines possess different cytoplasmic compositions due to the cytoplasmic difference of the two individual cybrids. Hu et al. (2002) found that all four sterile plants derived from a sterile cybrid displayed the S. arvensis restriction pattern for both atp9 and coxII probes, whereas two fertile plants derived from a fertile cybrid displayed different restriction patterns: one with a B. napus pattern for coxII and S. arvensis pattern for atp9, the other one with a B. napus pattern for both probes. Obviously rearrangement of the mitochondrial genome occurred even at early generations in offspring of the fertile cybrid. Homologous recombination between large repeat sequences helps to drive the creation of multipartite structures consisting of varied sub-genomic molecules, being a driving force in plant mitochondrial genome evolution (Albert et al. 1998). In the fertile plant with a mixed RFLP pattern, it is likely that the whole or part of the mitochondrial genome of both parental lines was present, providing a high chance for rearrangement. Therefore, selection of fertile lines with fertility restoration from offspring of the fertile plant should allow a possibility of mitochondrial genome rearrangement. Feng et al. (2009) found that pearl millet CMS systems prone to spontaneous fertility reversion experienced sporadic mitochondrial genome instability. Barr et al. (2005) proposed that differential transmission of mitochondrial genes could occur by both differential replication of mitochondrial genomes in heteroplasmic cells and differential segregation of mitochondrial genomes during mitosis and meiosis. The specific subgenomic molecules seem to be unconstant among mtDNA mitotypes and throughout breeding generations.
As the restorer lines of Nsa CMS are additional lines with a pair of additional chromsomes from S. arvensis, and the restorer gene(s) is located on the additional chromosomes (Wei et al. 2010), it is impossible to study the inheritance of restoration and clone the restorer gene(s) by genetic mapping. Using transcriptome analysis, certain PPR-like and GA-related genes that were previously reported to be essential for floral development were identified specific to Nsa restorer lines and S. arvensis (Yan et al. 2013; Liu et al. 2014). Tissue-specific expression of these genes and transgenic complementation experiments are underway for the functional identification of these genes. In the future, to reveal the mechanisms underlying fertility regulation in the Nsa CMS system, the R and S gene(s) should be cloned, and interaction between R and S genes should be assessed. The identification of anther development processes and basic molecular characterization of Nsa CMS conducted in this research can be used as a foundation for the future identification of candidate genes involved in the CMS and restorer phenotypes, leading to future gene expression studies and gene cloning approaches.
References
Albert B, Godelle B, Gouyon PH (1998) Evolution of the plant mitochondrial genome: dynamics of duplication and deletion of sequences. J Mol Evol 46:155–158
Balk J, Leaver CJ (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13:1803–1818
Barr CM, Neiman M, Taylor DR (2005) Inheritance and recombination of mitochondrial genomes in plants, fungi and animals. New Phytol 168(1):39–50
Bereterbide A, Hernould M, Farbos I, Glimelius K, Mouras A (2002) Restoration of stamen development and production of functional pollen in an alloplasmic CMS tobacco line by ectopic expression of the Arabidopsis thaliana SUPERMAN gene. Plant J 29(5):1–10
Budar F, Pelletier G (2001) Male sterility in plants: occurrence, determinism, significance and use. C R Acad, Sci, III 324:543–550
Cheng JH, Li YC, Hu Q, Mei DS, Li YD, Xu YS (2008) Molecular identification and distinctness of Nsa male sterile cytoplasm in Brassica napus. Acta Agron Sin 34(11):1946–1952
Feng X, Kaur AP, Mackenzie SA, Dweikat IM (2009) Substoichiometric shifting in the fertility reversion of cytoplasmic male sterility pearl millet. Theor Appl Genet 118:1361–1370
Fu TD (1981) Production and research of rape seed in the People Republic of China. Eucarp ia Cruciferae Newslett 6:6–7
Gonzalez-Melendi P, Uyttewaal M, Morcillo CN, Mora JRH, Budar F, Lucas MM (2008) A light and electron microscopy analysis of the events leading to male sterility in Ogu-INRA CMS of rapeseed (Brassica napus). J Exp Bot 59(4):827–838
Hu Q, Andersen SB, Dixelius C, Hansen LN (2002) Production of fertile intergeneric somatic hybrids between B. napus and S. arvensis for the enrichment of rapeseed gene pool. Plant Cell Rep 21:147–152
Hu Q, Li YC, Mei DS, Fang XP, Hansen LN, Andersen SB (2003) Establishment and identification of cytoplasmic male sterility in Brassica napus by intergeneric somatic hybridization. Agric Sci China 2(12):1321–1328
Kadowaki K, Suzuki T, Kazama S (1990) A chimeric gene containing the 50 portion of atp6 is associated with cytoplasmic malesterility of rice. Mol Gen Genet 224:10–16
Kaul MLH (1988) Male sterility in higher plants. Springer, Berlin, pp 3–13
Kini AV, Seetharam A, Joshi SS (1994) Mechanism of pollen abortion in cytoplasmic male lines of sunflower. Cytologia 38:425–454
Liu Y, Dong ZS, Zhang GS, Dong JG, Liu CS, Liu XX, Li HB (2005) Cytological Study on Growth of Anther of CMS 212A in Brassica napus L. Acta Agric Boreali-Occident Sin 14(1):33–37
Liu J, Mei DS, Li YC, Huang SM, Hu Q (2014) Deep RNA-Seq to unlock the gene bank of floral development in Sinapis arvensis. PLoS One 9(9):e105775
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rousselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferea by protoplast fusion. Mol Gen Genet 191:244–250
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16:S46–S60
Uzunova MI, Ecke W, Weißleder K, Röbbelen G (1995) Mapping the genome of rapeseed (Brassica napus L.) I. Construction of an RFLP linkage map and localisation of QTLs for seed glucosinolate content. Theor Appl Genet 90:194–204
Virmani SS, Edwards IB (1983) Current status and future prospects for breeding hybrid rice and wheat. Adv Agron 36:145–214
Wang WM, Li YC, Hu Q, Cheng JH, Mei DS, Hao JY (2008) An efficient protocol for pure mitochondrial DNA extraction from oilseed rape. Chin J Oil Crop Sci 30(3):357–360
Wei WL, Wang HZ, Liu GH (2005) Anatomical observations of anther development of NCa, a cytoplasmic male sterile line in rapeseed (Brassica napus L.). Sci Agric Sin 38(6):1232–1237
Wei WH, Li YC, Wang LJ, Liu SY, Yan XH, Mei DS, Li YD, Xu YS, Peng PF, Hu Q (2010) Development of a novel Sinapis arvensis disomic addition line in Brassica napus containing the restorer gene for Nsa CMS and improved resistance to Sclerotinia sclerotiorum and pod shattering. Theor Appl Genet 120(6):1089–1097
Shiga T, Baba S (1973) Cytoplasmic male sterility in oilseed rape (Brassica napus L.), and its utilization to breeding. Jpn J Breed 23(4):187–197
Yan XH, Dong CH, Yu JY, Liu WH, Jiang CH, Liu J, Hu Q, Fang XP, Wei WH (2013) Transcriptome profile analysis of young floral buds of fertile and sterile plants from the self-pollinated offspring of the hybrid between novel restorer line NR1 and Nsa CMS line in Brassica napus. BMC Genom 14:26
Yu FQ, Fu TD (1990) Cytomorphological research on anther development of several male sterile lines in Brassica napus L. J Wuhan Bot Res 8(3):209–216
Zhou YM, Fu TD (2007) Genetic improvement of rapeseed in China. In: Proceedings of the 12th international rapeseed congress. Wuhan, I: 2–4
Acknowledgments
We wish to acknowledge the many useful comments made by the referees of the journal. This research was funded by the Natural Science Foundation of China (30871553 and 31000725). ASM is supported by an Australian Research Council Discovery Early Career Researcher Award (DE120100668).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, J., Xiang, R., Wang, W. et al. Cytological and molecular analysis of Nsa CMS in Brassica napus L. . Euphytica 206, 279–286 (2015). https://doi.org/10.1007/s10681-015-1443-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1443-y