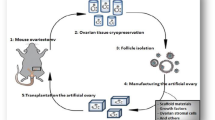
Abstract
In recent years, reproductive medicine has made good use of tissue engineering and regenerative medicine techniques to develop alternatives to restore fertility in cancer patients. For young female cancer patients who cannot undergo any of the currently applied strategies due to the possible presence of malignant cells in their ovaries, the challenge is creating an in vitro or in vivo artificial ovary using carefully selected biomaterials. Thanks to its numerous qualities, fibrin has been widely used as a scaffold material for fertility preservation applications. The goal of this review is to examine and discuss the applications and advantages of this biopolymer for fertility restoration in cancer patients, and consider the main results achieved so far.







Similar content being viewed by others
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- AMH:
-
Anti-Müllerian hormone
- Bfgf:
-
Basic fibroblast growth factor
- Ca2+ :
-
Calcium ions
- ECM:
-
Extracellular matrix
- F/T:
-
Fibrinogen (mg/mL) and thrombin (IU/mL) concentrations
- FDA:
-
Food and Drug Administration
- Fibrin/HBP/VEGF:
-
Fibrin/heparin-binding peptide/vascular endothelial growth factor
- FpA:
-
Fibrinogen peptide A
- FpB:
-
Fibrinogen peptide B
- FSH:
-
Follicle-stimulating hormone
- FXIII:
-
Factor thirteen
- HA:
-
Hyaluronic acid
- IVC:
-
In vitro culture
- IVG:
-
In vitro growth
- PL:
-
Platelet lysate
- RGD:
-
Arginine-glycine-aspartate
- TAO:
-
Transplantable artificial ovary
- tPA:
-
Tissue plasminogen activator
- VEGF:
-
Vascular endothelial growth factor
References
Ahmed, E. M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 6:105–121, 2015.
Albala, D. Fibrin sealants in clinical practice. Cardiovasc. Surg. 11:5–11, 2003.
Amorim, C. A. Artificial Ovary. In: Gonadal Tissue Cryopreservation in Fertility Preservation, edited by N. Suzuki, and J. Donnez. Tokyo: Springer, 2016, pp. 175–192.
Amorim, C. A., A. Van Langendonckt, A. David, M. M. Dolmans, and J. Donnez. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum. Reprod. 24:92–99, 2009.
Atala, A. Tissue engineering of reproductive tissues and organs. Fertil. Steril. 98:21–29, 2012.
Bedoschi, G., P. A. Navarro, and K. Oktay. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Fut. Oncol. 12:2333–2344, 2016.
Brito, I. R., G. M. Silva, A. D. Sales, C. H. Lobo, G. Q. Rodrigues, R. F. Sousa, A. Moura, C. Calderon, M. Bertolini, C. C. Campello, J. Smitz, and J. R. Figueiredo. Fibrin-alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod. Domest. Anim. 51:997–1009, 2016.
Brown, A. C., and T. H. Barker. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 10:1502–1514, 2014.
Brown, L. F., N. Lanir, J. McDonagh, K. Tognazzi, A. M. Dvorak, and H. F. Dvorak. Fibroblast migration in fibrin gel matrices. Am. J. Pathol. 142:273–283, 1993.
Buchta, C., H. C. Hedrich, M. Macher, P. Hocker, and H. Redl. Biochemical characterization of autologous fibrin sealants produced by CryoSeal and Vivostat in comparison to the homologous fibrin sealant product Tissucol/Tisseel. Biomaterials 26:6233–6241, 2005.
Caló, E., and V. V. Khutoryanskiy. Biomedical applications of hydrogels: a review of patents and commercial products. Eur. Polymer J. 65:252–267, 2015.
Catelas, I., N. Sese, B. M. Wu, J. C. Dunn, S. Helgerson, and B. Tawil. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 12:2385–2396, 2006.
Chiti, M. C., M. M. Dolmans, R. Orellana, M. Soares, F. Paulini, J. Donnez, and C. A. Amorim. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum. Reprod. 31:427–435, 2016.
Chiti, M. C. D. M., C. M. Lucci, F. Paulini, J. Donnez, and C. A. Amorim. Further insight into the impact of mouse follicle stage on graft outcome in an artificial ovary environment. 2016.
Choi, J. K., P. Agarwal, H. Huang, S. Zhao, and X. He. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 35:5122–5128, 2014.
Cronkite, E. P., E. L. Lozner, and J. M. Deaver. Use of thrombin and fibrinogen in skin grafting: preliminary report. J. Am. Med. Assoc. 124:976–978, 1944.
Dath, C., A. Dethy, A. Van Langendonckt, A. S. Van Eyck, C. A. Amorim, V. Luyckx, J. Donnez, and M. M. Dolmans. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum. Reprod. 26:1431–1439, 2011.
Dolmans, M. M., V. Luyckx, J. Donnez, C. Y. Andersen, and T. Greve. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 99:1514–1522, 2013.
Dolmans, M. M., B. Martinez-Madrid, E. Gadisseux, Y. Guiot, W. Y. Yuan, A. Torre, A. Camboni, A. Van Langendonckt, and J. Donnez. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 134:253–262, 2007.
Dolmans, M. M., N. Michaux, A. Camboni, B. Martinez-Madrid, A. Van Langendonckt, S. A. Nottola, and J. Donnez. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum. Reprod. 21:413–420, 2006.
Donnez, J., and M. M. Dolmans. Fertility preservation in women. Nat. Rev. Endocrinol. 9:735–749, 2013.
Donnez, J., and M. M. Dolmans. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J. Assist. Reprod. Genet. 32:1167–1170, 2015.
Donnez, J., M. M. Dolmans, A. Pellicer, C. Diaz-Garcia, M. Sanchez Serrano, K. T. Schmidt, E. Ernst, V. Luyckx, and C. Y. Andersen. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil. Steril. 99:1503–1513, 2013.
Duong, H., B. Wu, and B. Tawil. Modulation of 3D fibrin matrix stiffness by intrinsic fibrinogen-thrombin compositions and by extrinsic cellular activity. Tissue Eng. Part A 15:1865–1876, 2009.
Falvo, M. R., O. V. Gorkun, and S. T. Lord. The molecular origins of the mechanical properties of fibrin. Biophys. Chem. 152:15–20, 2010.
Gao, J. M., J. Yan, R. Li, M. Li, L. Y. Yan, T. R. Wang, H. C. Zhao, Y. Zhao, Y. Yu, and J. Qiao. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum. Reprod. 28:2784–2793, 2013.
Gosden, R. G. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum. Reprod. 5:117–122, 1990.
Gougeon, A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 17:121–155, 1996.
Guthold, M., W. Liu, E. A. Sparks, L. M. Jawerth, L. Peng, M. Falvo, R. Superfine, R. R. Hantgan, and S. T. Lord. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem. Biophys. 49:165–181, 2007.
Hawiger, J. Formation and regulation of platelet and fibrin hemostatic plug. Hum. Pathol. 18:111–122, 1987.
Hornick, J. E., F. E. Duncan, L. D. Shea, and T. K. Woodruff. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod. 27:1801–1810, 2012.
Jadoul, P., and J. Donnez. How does bone marrow transplantation affect ovarian function and fertility? Curr. Opin. Obstet. Gynecol. 24:164–171, 2012.
Janmey, P. A., J. P. Winer, and J. W. Weisel. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 6:1–10, 2009.
Jensen, J. R., D. E. Morbeck, and C. C. Coddington, 3rd. Fertility preservation. Mayo Clin. Proc. 86:45–49, 2011.
Jin, S. Y., L. Lei, A. Shikanov, L. D. Shea, and T. K. Woodruff. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil. Steril. 93:2633–2639, 2010.
Joch, C. The safety of fibrin sealants. Cardiovasc. Surg. 11:23–28, 2003.
Kang, B. J., Y. Wang, L. Zhang, Z. Xiao, and S. W. Li. bFGF and VEGF improve the quality of vitrified-thawed human ovarian tissues after xenotransplantation to SCID mice. J. Assist. Reprod. Genet. 33:281–289, 2016.
Kniazeva, E., A. N. Hardy, S. A. Boukaidi, T. K. Woodruff, J. S. Jeruss, and L. D. Shea. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep. 5:17709, 2015.
Laronda, M. M., A. E. Jakus, K. A. Whelan, J. A. Wertheim, R. N. Shah, and T. K. Woodruff. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50:20–29, 2015.
Luyckx, V., M. M. Dolmans, J. Vanacker, C. Legat, C. Fortuno Moya, J. Donnez, and C. A. Amorim. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 101:1149–1156, 2014.
Luyckx, V., M. M. Dolmans, J. Vanacker, S. R. Scalercio, J. Donnez, and C. A. Amorim. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res. 6:83, 2013.
Makris, M., J. A. Garson, C. J. Ring, P. W. Tuke, R. S. Tedder, and F. E. Preston. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 81:1898–1902, 1993.
Marx, G. Evolution of fibrin glue applicators. Transfus. Med. Rev. 17:287–298, 2003.
Mooney, D. M. A. The promise of tissue engineering. In: Scientific American 1999, pp. 37–43.
Mosesson, M. W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 3:1894–1904, 2005.
Mosesson, M. W., K. R. Siebenlist, and D. A. Meh. The structure and biological features of fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 936:11–30, 2001.
Mott, J. D., and Z. Werb. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 16:558–564, 2004.
Onofre, J., Y. Baert, K. Faes, and E. Goossens. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum. Reprod. Update 22:744–761, 2016.
Palta, S., R. Saroa, and A. Palta. Overview of the coagulation system. Indian J. Anaesth. 58:515–523, 2014.
Paulini, F., J. M. Vilela, M. C. Chiti, J. Donnez, P. Jadoul, M. M. Dolmans, and C. A. Amorim. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. Biomed. Online 33:425–432, 2016.
Rajabzadeh, A. R., H. Eimani, H. Mohseni Koochesfahani, A. H. Shahvardi, and R. Fathi. Morphological study of isolated ovarian preantral follicles using fibrin gel plus platelet lysate after subcutaneous transplantation. Cell J. 17:145–152, 2015.
Rampichova, M., E. Filova, F. Varga, A. Lytvynets, E. Prosecka, L. Kolacna, J. Motlik, A. Necas, L. Vajner, J. Uhlik, and E. Amler. Fibrin/hyaluronic acid composite hydrogels as appropriate scaffolds for in vivo artificial cartilage implantation. ASAIO J. 56:563–568, 2010.
Rowe, S. L., and J. P. Stegemann. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules 7:2942–2948, 2006.
Schwartz, M. L., S. V. Pizzo, R. L. Hill, and P. A. McKee. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J. Biol. Chem. 248:1395–1407, 1973.
Shikanov, A., M. Xu, T. K. Woodruff, and L. D. Shea. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 30:5476–5485, 2009.
Shikanov, A., Z. Zhang, M. Xu, R. M. Smith, A. Rajan, T. K. Woodruff, and L. D. Shea. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. Part A 17:3095–3104, 2011.
Siebenlist, K. R., D. A. Meh, and M. W. Mosesson. Plasma factor XIII binds specifically to fibrinogen molecules containing gamma chains. Biochemistry 35:10448–10453, 1996.
Smith, M. A., S. F. Altekruse, P. C. Adamson, G. H. Reaman, and N. L. Seibel. Declining childhood and adolescent cancer mortality. Cancer 120:2497–2506, 2014.
Smith, R. M., A. Shikanov, E. Kniazeva, D. Ramadurai, T. K. Woodruff, and L. D. Shea. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng. Part A 20:3021–3030, 2014.
Smitz, J., M. M. Dolmans, J. Donnez, J. E. Fortune, O. Hovatta, K. Jewgenow, H. M. Picton, C. Plancha, L. D. Shea, R. L. Stouffer, E. E. Telfer, T. K. Woodruff, and M. B. Zelinski. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum. Reprod. Update 16:395–414, 2010.
Soares, M., K. Sahrari, C. A. Amorim, P. Saussoy, J. Donnez, and M. M. Dolmans. Evaluation of a human ovarian follicle isolation technique to obtain disease-free follicle suspensions before safely grafting to cancer patients. Fertil. Steril. 104:672–680.e672, 2015.
Soares, M., K. Sahrari, M. C. Chiti, C. A. Amorim, J. Ambroise, J. Donnez, and M. M. Dolmans. The best source of isolated stromal cells for the artificial ovary: medulla or cortex, cryopreserved or fresh? Hum. Reprod. 30:1589–1598, 2015.
Soares, M., P. Saussoy, K. Sahrari, C. A. Amorim, J. Donnez, and M. M. Dolmans. Is transplantation of a few leukemic cells inside an artificial ovary able to induce leukemia in an experimental model? J. Assist. Reprod. Genet. 32:597–606, 2015.
Songsasen, N., C. Guzy, and D. E. Wildt. 121 Alginate-fibrin gel matrix promotes in vitro growth of dog secondary follicles. Reprod. Fertil. Dev. 24:173, 2011.
Stadler, P. H. G., R.-C. Koch, and C. Golker. Application of safety measures in the production of trasylol concerning bovine spongiform encephalopathy. J. Biotechnol. Healthc. 3:61–69, 1996.
Tamadon, A., K.-H. Park, Y. Y. Kim, B.-C. Kang, and S.-Y. Ku. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng. Regen. Med. 13:447–454, 2016.
Telfer, E. E., and M. B. Zelinski. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil. Steril. 99:1523–1533, 2013.
Thomson, K. S., S. K. Dupras, C. E. Murry, M. Scatena, and M. Regnier. Proangiogenic microtemplated fibrin scaffolds containing aprotinin promote improved wound healing responses. Angiogenesis 17:195–205, 2014.
Torrance, C., E. Telfer, and R. G. Gosden. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J. Reprod. Fertil. 87:367–374, 1989.
Turan, V., and K. Oktay. Sexual and fertility adverse effects associated with chemotherapy treatment in women. Expert Opin. Drug Saf. 13:775–783, 2014.
Van der Ven, H., J. Liebenthron, M. Beckmann, B. Toth, M. Korell, J. Krussel, T. Frambach, M. Kupka, M. K. Hohl, K. Winkler-Crepaz, S. Seitz, A. Dogan, G. Griesinger, F. Haberlin, M. Henes, R. Schwab, M. Sutterlin, M. von Wolff, R. Dittrich, and P. N. Ferti. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum. Reprod. 31:2031–2041, 2016.
Van Eyck, A. S., B. F. Jordan, B. Gallez, J. F. Heilier, A. Van Langendonckt, and J. Donnez. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil. Steril. 92:374–381, 2009.
Vanacker, J., M. M. Dolmans, V. Luyckx, J. Donnez, and C. A. Amorim. First transplantation of isolated murine follicles in alginate. Regen. Med. 9:609–619, 2014.
Vanacker J., A. Camboni, C. Dath, A. Van Langendonckt, M. M. Dolmans, J. Donnez, and C. A. Amorim. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil. Steril. 96:379–383.e373, 2011.
Vanacker, J., V. Luyckx, M. M. Dolmans, A. Des Rieux, J. Jaeger, A. Van Langendonckt, J. Donnez, and C. A. Amorim. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 33:6079–6085, 2012.
Waimey, K. E., F. E. Duncan, H. I. Su, K. Smith, H. Wallach, K. Jona, C. Coutifaris, C. R. Gracia, L. D. Shea, R. E. Brannigan, R. J. Chang, M. B. Zelinski, R. L. Stouffer, R. L. Taylor, and T. K. Woodruff. Future directions in oncofertility and fertility preservation: a report from the 2011 oncofertility consortium conference. J. Adolesc. Young Adult Oncol. 2:25–30, 2013.
Wallace, W. H., T. W. Kelsey, and R. A. Anderson. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil. Steril. 105:6–12, 2016.
Weisel, J. W. Fibrinogen and fibrin. Adv. Protein Chem. 70:247–299, 2005.
Wolberg, A. S. Thrombin generation and fibrin clot structure. Blood Rev. 21:131–142, 2007.
Woodruff, T. K., and L. D. Shea. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J. Assist. Reprod. Genet. 28:3–6, 2011.
Xu, M., S. L. Barrett, E. West-Farrell, L. A. Kondapalli, S. E. Kiesewetter, L. D. Shea, and T. K. Woodruff. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 24:2531–2540, 2009.
Xu, M., A. T. Fazleabas, A. Shikanov, E. Jackson, S. L. Barrett, J. Hirshfeld-Cytron, S. E. Kiesewetter, L. D. Shea, and T. K. Woodruff. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol. Reprod. 84:689–697, 2011.
Xu, M., P. K. Kreeger, L. D. Shea, and T. K. Woodruff. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 12:2739–2746, 2006.
Xu, J., M. S. Lawson, R. R. Yeoman, T. A. Molskness, A. Y. Ting, R. L. Stouffer, and M. B. Zelinski. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum. Reprod. 28:2187–2200, 2013.
Zhang, X., L. Li, Y. Bai, R. Shi, H. Wei, and S. Zhang. Mouse undifferentiated spermatogonial stem cells cultured as aggregates under simulated microgravity. Andrologia 46:1013–1021, 2014.
Zhou, H., M. A. Malik, A. Arab, M. T. Hill, and A. Shikanov. Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLoS ONE 10:e0140205, 2015.
Zhu, J., and R. E. Marchant. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 8:607–626, 2011.
Acknowledgments
The authors thank Mira Hryniuk, BA, for reviewing the English language of the manuscript. This study was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (FNRS) (Grant 5/4/150/5 awarded to Marie-Madeleine Dolmans and FRS-FNRS funding for C. A. Amorim as research associate) and donations from the Ferrero family.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Chiti, M.C., Dolmans, M.M., Donnez, J. et al. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann Biomed Eng 45, 1650–1663 (2017). https://doi.org/10.1007/s10439-017-1817-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1817-5