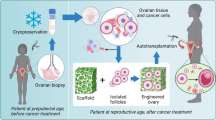
Abstract
Various gynecologic diseases and chemoradiation or surgery for the management of gynecologic malignancies may damage the uterus and ovaries, leading to clinical problems such as infertility or early menopause. Embryo or oocyte cryopreservation—the standard method for fertility preservation—is not a feasible option for patients who require urgent treatment because the procedure requires ovarian stimulation for at least several days. Hormone replacement therapy (HRT) for patients diagnosed with premature menopause is contraindicated for patients with estrogen-dependent tumors or a history of thrombosis. Furthermore, these methods cannot restore the function of the uterus and ovaries. Although autologous transplantation of cryopreserved ovarian tissue is being attempted, it may re-introduce malignant cells after cancer treatment. With the recent development in regenerative medicine, research on engineered biomaterials for the restoration of female reproductive organs is being actively conducted. The use of engineered biomaterials is a promising option in the field of reproductive medicine because it can overcome the limitations of current therapies. Here, we review the ideal properties of biomaterials for reproductive tissue engineering and the recent advancements in engineered biomaterials for the regeneration of female reproductive organs.



Similar content being viewed by others
Data Availability
Not applicable.
References
Group, S.G.O.C.P.E.C.W, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol. 2014;134(2):393–402.
Vassilakopoulou M, Boostandoost E, Papaxoinis G, de la Motte Rouge T, Khayat D, Psyrri A. Anticancer treatment and fertility: effect of therapeutic modalities on reproductive system and functions. Crit Rev Oncol Hematol. 2016;97:328–34.
Vitale SG, la Rosa VL, Rapisarda AMC, Laganà AS. The importance of fertility preservation counseling in patients with gynecologic cancer. J Reprod Infertil. 2017;18(2):261–3.
Salama M, Mallmann P. Emergency fertility preservation for female patients with cancer: clinical perspectives. Anticancer Res. 2015;35(6):3117–27.
Kim H, Kim H, Ku SY. Fertility preservation in pediatric and young adult female cancer patients. Ann Pediatr Endocrinol Metab. 2018;23(2):70–4.
Blagden S, et al. The effect of early pregnancy following chemotherapy on disease relapse and foetal outcome in women treated for gestational trophoblastic tumours. Br J Cancer. 2002;86(1):26–30.
Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104(5):1097–8.
Lee S, et al. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int J Mol Sci. 2019:20(13).
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–10.
Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–65.
Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–11.
Practice, C.o.G. Committee Opinion No. 698: Hormone therapy in primary ovarian insufficiency. Obstet Gynecol. 2017;129(5):e134.
Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. Jama. 2003;289(24):3243–53.
Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, et al. Estrogen Plus Progestin Risk Ven Thromb. Jama. 2004;292(13):1573–80.
Iavazzo C, Gkegkes ID. Possible role of DaVinci Robot in uterine transplantation. J Turk Ger Gynecol Assoc. 2015;16(3):179–80.
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 1999;140(1):462–71.
David A, Day JR, Cichon AL, Lefferts A, Cascalho M, Shikanov A. Restoring ovarian endocrine function with encapsulated ovarian allograft in immune competent mice. Ann Biomed Eng. 2017;45(7):1685–96.
Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17(23-24):3095–104.
Tanaka A, Nakamura H, Tabata Y, Fujimori Y, Kumasawa K, Kimura T. Effect of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogels on frozen-thawed human ovarian tissue in a xenograft model. J Obstet Gynaecol Res. 2018;44(10):1947–55.
Miki F, Maruyama T, Miyazaki K, Takao T, Yoshimasa Y, Katakura S, et al. The orientation of a decellularized uterine scaffold determines the tissue topology and architecture of the regenerated uterus in rats. Biol Reprod. 2019;100(5):1215–27.
Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35(31):8791–800.
Jiang WC, Cheng YH, Yen MH, Chang Y, Yang VW, Lee OK. Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomaterials. 2014;35(11):3607–17.
Santos JM, et al. Immunohistochemical localization of fibroblast growth factor-2 in the sheep ovary and its effects on pre-antral follicle apoptosis and development in vitro. Reprod Domest Anim. 2014;49(3):522–8.
Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475.
Kim YJ, Kim YY, Kim DW, Joo JK, Kim H, Ku SY. Profile of MicroRNA expression in endometrial cell during in vitro culture according to progesterone concentration. Tissue Eng Regen Med. 2017;14(5):617–29.
Tamadon A, Park KH, Kim YY, Kang BC, Ku SY. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng Regen Med. 2016;13(5):447–54.
Black J. Biological performance of materials : fundamentals of biocompatibility. 4th ed. Boca Raton: CRC Taylor & Francis; 2006. p. 497.
Martin JR, Gupta MK, Page JM, Yu F, Davidson JM, Guelcher SA, et al. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014;35(12):3766–76.
Baah-Dwomoh A, McGuire J, Tan T, de Vita R. Mechanical properties of female reproductive organs and supporting connective tissues: a review of the current state of knowledge. Appl Mech Rev. 2016;68(6):060801.
Chen PY, et al. Structure and mechanical properties of selected biological materials. J Mech Behav Biomed Mater. 2008;1(3):208–26.
Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther. 2018;9:252.
Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, Moon SY, et al. Effect of gonadotropins on human endometrial stromal cell proliferation in vitro. Arch Gynecol Obstet. 2002;266(4):223–8.
Alshaikh AB, Padma AM, Dehlin M, Akouri R, Song MJ, Brännström M, et al. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J Ovarian Res. 2019;12(1):58.
Wood CD, Vijayvergia M, Miller FH, Carroll T, Fasanati C, Shea LD, et al. Multi-modal magnetic resonance elastography for noninvasive assessment of ovarian tissue rigidity in vivo. Acta Biomater. 2015;13:295–300.
Engelmayr GC Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7(12):1003–10.
Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28(1):3–6.
Zhang Y, An D, Pardo Y, Chiu A, Song W, Liu Q, et al. High-water-content and resilient PEG-containing hydrogels with low fibrotic response. Acta biomaterialia. 2017;53:100–8.
Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2010;93(3):941–53.
Hillel AT, et al. Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans. Sci Transl Med. 2011;3(93):93ra67.
Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6(5):385–92.
Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41(11):3993–4004.
Chiu YC, Kocagöz S, Larson JC, Brey EM. Evaluation of physical and mechanical properties of porous poly (ethylene glycol)-co-(L-lactic acid) hydrogels during degradation. PLoS ONE. 2013;8(4):e60728.
Artzi N, Oliva N, Puron C, Shitreet S, Artzi S, bon Ramos A, et al. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging (vol 10, pg 704, 2011). Nat Mater. 2011;10(11):896.
Lee BK, Yun Y, Park K. PLA micro- and nano-particles. Adv Drug Deliv Rev. 2016;107:176–91.
Meng CX, Andersson KL, Bentin-Ley U, Gemzell-Danielsson K, Lalitkumar PGL. Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human endometrial cell culture model. Fertil Steril. 2009;91(1):256–64.
Zhou H, Malik MA, Arab A, Hill MT, Shikanov A. Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLoS One. 2015;10(10):e0140205.
Zhang SS, Xu XX, Xiang WW, Zhang HH, Lin HL, Shen LE, et al. Using 17β-estradiol heparin-poloxamer thermosensitive hydrogel to enhance the endometrial regeneration and functional recovery of intrauterine adhesions in a rat model. The FASEB Journal. 2020;34(1):446–57.
Yao Q, Zheng YW, Lan QH, Wang LF, Huang ZW, Chen R, et al. Aloe/poloxamer hydrogel as an injectable β-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur J Pharm Sci. 2020;148:105316.
Wenbo Q, Lijian X, Shuangdan Z, Jiahua Z, Yanpeng T, Xuejun Q, et al. Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int J Biol Macromol. 2020;143:163–72.
Paul K, Darzi S, McPhee G, del Borgo MP, Werkmeister JA, Gargett CE, et al. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019;97:162–76.
Paul K, et al. 3D bioprinted endometrial stem cells on melt electrospun PCL meshes for pelvic floor application promote anti-inflammatory responses in mice. Available at SSRN. 2019;3387674.
Kim YY, Park KH, Kim YJ, Kim MS, Liu HC, Rosenwaks Z, et al. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019;89:139–51.
Bus A, van Hoeck V, Langbeen A, Leroy JLMR, Bols PEJ. Effects of vitrification on the viability of alginate encapsulated isolated bovine pre-antral follicles. J Assist Reprod Genet. 2018;35(7):1187–99.
Day JR, David A, Cichon AL, Kulkarni T, Cascalho M, Shikanov A. Immunoisolating poly(ethylene glycol) based capsules support ovarian tissue survival to restore endocrine function. J Biomed Mater Res A. 2018;106(5):1381–9.
Pors SE, Ramløse M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, et al. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod. 2019;34(8):1523–35.
Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–9.
Mirzaeian L, et al. Optimizing the cell seeding protocol to human decellularized ovarian scaffold: application of dynamic system for bio-engineering. Cell J (Yakhteh). 2020;22(2).
Jamalzaei P, Valojerdi MR, Montazeri L, Baharvand H. Effects of alginate concentration and ovarian cells on in vitro development of mouse preantral follicles: a factorial study. Int J Fertil Steril. 2020;13(4):330–8.
Nagashima JB, Wildt DE, Travis AJ, Songsasen N. Activin promotes growth and antral cavity expansion in the dog ovarian follicle. Theriogenology. 2019;129:168–77.
Sittadjody S, Saul JM, McQuilling JP, Joo S, Register TC, Yoo JJ, et al. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat Commun. 2017;8(1):1858.
Laronda MM, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8(1):1–10.
Kim YY, Tamadon A, Ku SY. Potential use of antiapoptotic proteins and noncoding RNAs for efficient in vitro follicular maturation and ovarian bioengineering. Tissue Eng Part B Rev. 2017;23(2):142–58.
Gilula NB, Epstein ML, Beers WH. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol. 1978;78(1):58–75.
West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25(4):287–99.
Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A, et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119.
Park KE, Kim YY, Ku SY, Baek SM, Huh Y, Kim YJ, et al. Effects of alginate hydrogels on in vitro maturation outcome of mouse preantral follicles. Tissue Eng Regen Med. 2012;9(3):170–4.
Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53.
Park KE, Ku SY, Jung KC, Liu HC, Kim YY, Kim YJ, et al. Effects of urinary and recombinant gonadotropins on in vitro maturation outcomes of mouse preantral follicles. Reprod Sci. 2013;20(8):909–16.
Kim YJ, Kim YY, Kang BC, Kim MS, Ko IK, Liu HC, et al. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J Tissue Eng Regen Med. 2017;11(11):3100–10.
Kim YJ, Ku SY, Kim YY, Liu HC, Chi SW, Kim SH, et al. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod. 2013;28(11):3050–61.
Kim YJ, Ku SY, Rosenwaks Z, Liu HC, Chi SW, Kang JS, et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod Sci. 2010;17(12):1081–9.
Kim YJ, Park KE, Kim YY, Kim H, Ku SY, Suh CS, et al. Effects of estradiol on the paracrine regulator expression of in vitro maturated murine ovarian follicles. Tissue Eng Regen Med. 2017;14(1):31–8.
Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81(3):587–94.
Camboni A, van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology. 2013;67(1):64–9.
King SM, et al. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. J Vis Exp. 2011;52.
Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31(8):1013–28.
Vanacker J, Amorim CA. Alginate: a versatile biomaterial to encapsulate isolated ovarian follicles. Ann Biomed Eng. 2017;45(7):1633–49.
Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, van Langendonckt A, et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–85.
Ahn JI, Kim GA, Kwon HS, Ahn JY, Hubbell JA, Song YS, et al. Culture of preantral follicles in poly(ethylene) glycol-based, three-dimensional hydrogel: a relationship between swelling ratio and follicular developments. J Tissue Eng Regen Med. 2015;9(3):319–23.
Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet. 2013;30(10):1279–88.
Brito IR, Silva GM, Sales AD, Lobo CH, Rodrigues GQ, Sousa RF, et al. Fibrin-alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod Domest Anim. 2016;51(6):997–1009.
Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93(8):2633–9.
Wang T-r, et al. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. Hum Reprod. 2014;29(3):568–76.
Gao J, Huang Y, Li M, Zhao H, Zhao Y, Li R, et al. Effect of local basic fibroblast growth factor and vascular endothelial growth factor on subcutaneously allotransplanted ovarian tissue in ovariectomized mice. PLoS One. 2015;10(7):e0134035.
Felder S, Masasa H, Orenbuch A, Levaot N, Shachar Goldenberg M, Cohen S. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials. 2019;205:11–22.
Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, et al. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A. 2012;18(11-12):1229–38.
Mendez U, Zhou H, Shikanov A. Synthetic PEG hydrogel for engineering the environment of ovarian follicles. Methods Mol Biol. 2018;1758:115–28.
Kim J, Perez AS, Claflin J, David A, Zhou H, Shikanov A. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. NPJ Regen Med. 2016;1.
Buckenmeyer MJ, et al. Bioengineering an in situ ovary (ISO) for fertility preservation. bioRxiv. 2020.
Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Curry MA, Prentice MA, et al. OPTN/SRTR 2016 annual data report: pancreas. Am J Transplant. 2018;18:114–71.
David A, Day J, Shikanov A. Immunoisolation to prevent tissue graft rejection: current knowledge and future use. Exp Biol Med (Maywood). 2016;241(9):955–61.
Rios PD, Kniazeva E, Lee HC, Xiao S, Oakes RS, Saito E, et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng. 2018;115(8):2075–86.
Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JAV, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011.
Beral V, Bull D, Reeves G, Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–51.
Kim SW, Kim H, Ku SY, Suh CS, Kim SH, Choi YM. A successful live birth with in vitro fertilization and thawed embryo transfer after conservative treatment of recurrent endometrial cancer. Gynecol Endocrinol. 2018;34(1):15–9.
Sittadjody S, Enck KM, Wells A, Yoo JJ, Atala A, Saul JM, et al. Encapsulation of mesenchymal stem cells in 3D ovarian cell constructs promotes stable and long-term hormone secretion with improved physiological outcomes in a syngeneic rat model. Ann Biomed Eng. 2020;48(3):1058–70.
Araujo VR, et al. In vitro development of bovine secondary follicles in two- and three-dimensional culture systems using vascular endothelial growth factor, insulin-like growth factor-1, and growth hormone. Theriogenology. 2014;82(9):1246–53.
Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction. 2011;142(1):113–22.
Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28(8):2187–200.
Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84(4):689–97.
Cho E, Kim YY, Noh K, Ku SY. A new possibility in fertility preservation: the artificial ovary. J Tissue Eng Regen Med. 2019;13(8):1294–315.
Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–43.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–83.
Nakayama KH, Lee CCI, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8(5):e64134.
Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–41.
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–50.
Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–13.
Rosendahl M, Andersen MT, Ralfkiær E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94(6):2186–90.
Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–17.
Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20(11):2338–47.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20.
Mirzaeian L, Eivazkhani F, Hezavehei M, Moini A, Esfandiari F, Valojerdi MR, et al. Optimizing the cell seeding protocol to human decellularized ovarian scaffold: application of dynamic system for bio-engineering. Cell J. 2020;22(2):227–35.
Padma AM, Tiemann TT, Alshaikh AB, Akouri R, Song MJ, Hellström M. Protocols for rat uterus isolation and decellularization: applications for uterus tissue engineering and 3D cell culturing. Methods Mol Biol. 2018;1577:161–75.
Hellstrom M, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10(12):5034–42.
Baert Y, Goossens E. Preparation of scaffolds from decellularized testicular matrix. Methods Mol Biol. 2018;1577:121–7.
Rezaei Topraggaleh T, Rezazadeh Valojerdi M, Montazeri L, Baharvand H. A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomater Sci. 2019;7(4):1422–36.
Olalekan SA, Burdette JE, Getsios S, Woodruff TK, Kim JJ. Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod. 2017;96(5):971–81.
Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19(17-18):2045–62.
O’Neill JD, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96(3):1046–55 discussion 1055-6.
Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33(31):7756–64.
Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123(11):4950–62.
Oktay K, et al. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94 e1–9.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Updat. 2014;20(1):124–40.
Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N, et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev. 2010;16(3):341–50.
Pennarossa G, Ghiringhelli M, Gandolfi F, Brevini TAL. Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering. J Assist Reprod Genet. 2020;37(6):1329–39.
Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20.
Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16(8):2565–80.
Niklason L, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–93.
Mayorca-Guiliani AE, Madsen CD, Cox TR, Horton ER, Venning FA, Erler JT. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat Med. 2017;23(7):890–8.
Calle EA, Hill RC, Leiby KL, le AV, Gard AL, Madri JA, et al. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 2016;46:91–100.
Henning NF, LeDuc RD, Even KA, Laronda MM. Proteomic analyses of decellularized porcine ovaries identified new matrisome proteins and spatial differences across and within ovarian compartments. Sci Rep. 2019;9(1):20001.
Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, Leppert PC. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol Reprod. 2015;92(1):25.
Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261.
Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17(5):555–69.
Yun JW, Kim YY, Ahn JH, Kang BC, Ku SY. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng Regen Med. 2016;13(4):323–34.
Kim YY, Yun JW, Kim JM, Park CG, Rosenwaks Z, Liu HC, et al. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J Investig Med. 2016;64(4):888–93.
Brännström M, Johannesson L, Bokström H, Kvarnström N, Mölne J, Dahm-Kähler P, et al. Livebirth after uterus transplantation. The Lancet. 2015;385(9968):607–16.
Rogozea L, Sechel G, Fleancu A. Ethical aspects in bioengineering research. in WSEAS International Conference. Proceedings. Mathematics and Computers in Science and Engineering. World Sci Eng Acad Soc. 2009.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Updat. 1999;5(5):483–92.
Code Availability
Not applicable.
Funding
This study was supported by National Research Foundation of Korea (NRF) grant (2020R1A2C1010293) from Korean Government (MSIT) Fund and grant (No. 04-2015-0910) from the SNUH Research Fund.
Author information
Authors and Affiliations
Contributions
K.S.W: drafting the article, conception or design of the work, data collection, data analysis, and interpretation; K.Y.Y: drafting the article, critical revision of the article; K.H: data analysis and interpretation, final approval of the version to be published; Ku S.Y: data analysis and interpretation, conception or design of the work.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S.W., Kim, Y.Y., Kim, H. et al. Recent Advancements in Engineered Biomaterials for the Regeneration of Female Reproductive Organs. Reprod. Sci. 28, 1612–1625 (2021). https://doi.org/10.1007/s43032-021-00553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00553-y