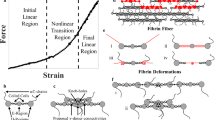
Abstract
In the past few years a great deal of progress has been made in studying the mechanical and structural properties of biological protein fibers. Here, we compare and review the stiffness (Young’s modulus, E) and breaking strain (also called rupture strain or extensibility, εmax) of numerous biological protein fibers in light of the recently reported mechanical properties of fibrin fibers. Emphasis is also placed on the structural features and molecular mechanisms that endow biological protein fibers with their respective mechanical properties. Generally, stiff biological protein fibers have a Young’s modulus on the order of a few Gigapascal and are not very extensible (εmax < 20%). They also display a very regular arrangement of their monomeric units. Soft biological protein fibers have a Young’s modulus on the order of a few Megapascal and are very extensible (εmax > 100%). These soft, extensible fibers employ a variety of molecular mechanisms, such as extending amorphous regions or unfolding protein domains, to accommodate large strains. We conclude our review by proposing a novel model of how fibrin fibers might achieve their extremely large extensibility, despite the regular arrangement of the monomeric fibrin units within a fiber. We propose that fibrin fibers accommodate large strains by two major mechanisms: (1) an α-helix to β-strand conversion of the coiled coils; (2) a partial unfolding of the globular C-terminal domain of the γ-chain.






Similar content being viewed by others
References
Liu, W., Jawerth, L. M., Sparks, E. A., Falvo, M. R., Hantgan, R. R., Superfine, R., Lord, S. T., & Guthold, M. (2006). Fibrin fibers have extraordinary extensibility and elasticity. Science, 313, 634.
Collet, J. P., Shuman, H., Ledger, R. E., Lee, S. T., & Weisel, J. W. (2005). The elasticity of an individual fibrin fiber in a clot. Proceedings of the National Academy of Sciences of the United States of America, 102, 9133.
Weisel, J. W. (1986). The electron-microscope band pattern of hman fibrin—various stains, lateral order, and carbohydrate localization. Journal of Ultrastructure and Molecular Structure Research, 96, 176.
Hantgan, R. R., Fowler, S. B., Erickson, H. P., & Hermans, J. (1980). Fibrin assembly: A comparison of electron microscopic and light scattering results. Thrombosis and Haemostasis, 44, 119.
Spraggon, G., Everse, S. J., & Doolittle, R. F. (1997). Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature, 389, 455.
Brown, J. H., Volkmann, N., Jun, G., Henschen-Edman, A. H., & Cohen, C. (2000). The crystal structure of modified bovine fibrinogen. Proceedings of the National Academy of Sciences of the United States of America, 97, 85.
Yang, Z., Kollman, J. M., Pandi, L., & Doolittle, R. F. (2001). Crystal structure of native chicken fibrinogen at 2.7 angstrom resolution. Biochemistry, 40, 12515.
Braaten, J. V., Jerome, W. G., & Hantgan, R. R. (1994). Uncoupling fibrin from integrin receptors Hastens fibrinolysis at the platelet-fibrin interface. Blood, 83, 982.
Doolittle, R. F. (2003). X-ray crystallographic studies on fibrinogen and fibrin. Journal of Thrombosis and Haemostasis, 1, 1559.
Williams, R. C. (1981). Morphology of bovine fibrinogen monomers and fibrin oligomers. Journal of Molecular Biology, 150, 399.
Hall, C. E., & Slayter, H. S. (1959). The fibrinogen molecule: its size, shape, and mode of polymerization. The Journal of Biophysical and Biochemical Cytology 5, 11.
Tsurupa, G., Tsonev, L., & Medved, L. (2002). Structural organization of the fibrin(ogen) alpha C-domain. Biochemistry, 41, 6449.
Doolittle, R. F., & Kollman, J. M. (2006). Natively unfolded regions of the vertebrate fibrinogen molecule. Proteins-Structure Function and Bioinformatics, 63, 391.
Burton, R. A., Tsurupa, G., Medved, L., & Tjandra, N. (2006). Identification of an ordered compact structure within the recombinant bovine fibrinogen alpha C-domain fragment by NMRT. Biochemistry, 45, 2257.
Yee, V. C., Pratt, K. P., Cote, H. C. F., LeTrong, I., Chung, D. W., Davie, E. W., Stenkamp, R. E., & Teller, D. C. (1997). Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure, 5, 125.
Everse, S. J., Spraggon, G., Veerapandian, L., & Doolittle, R. F. (1999). Conformational changes in fragments D and double-D from human fibrin(ogen) upon binding the peptide ligand Gly-His-Arg-Pro- amide. Biochemistry, 38, 2941.
Doolittle, R. F., Chen, A., & Pandi, L. (2006). Differences in binding specificity for the homologous gamma- and beta-chain “Holes” on fibrinogen: Exclusive binding of Ala-His-Arg-Pro-amide by the beta-chain hole. Biochemistry, 45, 13962.
Ferry, J. D. (1952). The mechanism of polymerization of fibrin. Proceedings of the National Academy of Sciences of the Untied States of America, 38, 566.
Hantgan, R. R., & Hermans, J. (1979). Assembly of Fibrin—Light-scattering study. Journal of Biological Chemistry, 254, 11272.
Ryan, E. A., Mockros, L. F., Weisel, J. W., & Lorand, L. (1999). Structural origins of fibrin clot rheology. Biophysical Journal, 77, 2813.
Carr, M. E., & Hermans, J. (1978). Size and density of fibrin fibers from turbidity. Macromolecules, 11, 46.
Blomback, B., Blomback, M., & Nillson, I. M. (1957). Coagulation studies on reptilase, an extract of the venom from Bothrops jararaca. Thrombosis et Diathesis Haemorrhagica, 1, 76.
Chen, R., & Doolittle, R. F. (1971). Gamma-gamma crosslinking sites in human and bovine fibrin. Biochemistry, 10, 15610.
McKee, P. A., Mattock, P., & Hill, R. L. (1970). Subunit structure of human fibrinogen, soluble fibrin, and cross-linked insoluble fibrin. Proceedings of the National Academy of Sciences of the United States of America, 66, 738.
Lorand, L. (2001). Factor XIII: Structure, activation, and interactions with fibrinogen and fibrin. Annals of the New York Academy of Sciences, 936, 291.
Sobel, J. H., & Gawinowicz, M. A. (1996). Identification of the alpha chain lysine donor sites involved in factor XIIIa fibrin cross-linking. The Journal of Biological Sciences, 271, 19288.
Matsuka, Y. V., Medved, L. V., Migliorini, M. M., & Ingham, K. C. (1996). Factor XIIIa-catalyzed cross-linking of recombinant alpha C fragments of human fibrinogen. Biochemistry, 35, 5810.
Murthy, S. N. P., Wilson, J. H., Lukas, T. J., Veklich, Y., Weisel, J. W., & Lorand, L. (2000). Transglutaminase-catalyzed crosslinking of the alpha and gamma constituent chains in fibrinogen. Proceedings of the National Academy of Sciences of the United States of America, 97, 44.
Doolittle, R. F. (2003). Structural basis of the fibrinogen-fibrin transformation: Contributions from X-ray crystallography. Blood Reviews, 17, 33.
Yang, Z., Mochalkin, I., & Doolittle, R. F. (2000). A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proceedings of the National Academy of Sciences of the United States of America, 97, 14156.
Caracciolo, G., De Spirito, M., Castellano, A. C., Pozzi, D., Amiconi, G., De Pascalis, A., Caminiti, R., & Arcovito, G. (2003). Protofibrils within fibrin fibres are packed together in a regular array. Thrombosis and Haemostasis, 89, 632.
Guthold, M., Liu, W., Stephens, B., Lord, S. T., Hantgan, R. R., Erie, D. A., Taylor, R. M., & Superfine, R. (2004). Visualization and mechanical manipulations of individual fibrin fibers suggest that fiber cross section has fractal dimension 1.3. Biophysical Journal, 87, 4226.
Brown, A. E. X., Litvinov, R. I., Discher, D. E., & Weisel, J. W. (2007). Forced Unfolding of Coiled-Coils in Fibrinogen by Single-Molecule AFM. Biophysical Journal, 92, L30.
Craig, C. L. (2003). Spiderwebs and silk. New York: Oxford University Press.
Gosline, J. M., Guerette, P. A., Ortlepp, C. S., & Savage, K. N. (1999). The mechanical design of spider silks: From fibroin sequence to mechanical function. Journal of Experimental Biology, 202, 3295.
Denny, M. (1976). Physical-properties of spiders silk and their role in design of orb-webs. Journal of Experimental Biology, 65, 483.
Termonia, Y. (1994). Molecular modeling of spider silk elasticity. Macromolecules, 27, 7378.
Hinman, M. B., Jones, J. A., & Lewis, R. V. (2000). Synthetic spider silk: A modular fiber. Trends in Biotechnology, 18, 374.
Hayashi, C. Y., & Lewis, R. V. (1998). Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. Journal of Molecular Biology, 275, 773.
Gosline, J. M. (1980). The elastic properties of rubber-like proteins and highly extensible tissues Cambridge: Cambridge University Press.
Aaron, B. B., & Gosline, J. M. (1981). Elastin as a random-network elastomer—a mechanical and optical analysis of single elastin fibers. Biopolymers, 20, 1247.
Hoeve, C. A. J., & Flory, P. J. (1974). Elastic properties of elastin. Biopolymers, 13, 677.
Urry, D. W., Hugel, T., Seitz, M., Gaub, H. E., Sheiba, L., Dea, J., Xu, J., & Parker, T. (2002). Elastin: A representative ideal protein elastomer. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 357, 169.
Li, B., & Daggett, V. (2002). Molecular basis for the extensibility of elastin. Journal of Muscle Research and Cell Motility, 23, 561.
Rosenbloom, J., Abrams, W. R., & Mecham, R. (1993). Extracellular-matrix. 4. The Elastic Fiber. Faseb Journal, 7, 1208.
Gosline, J., Lillie, M., Carrington, E., Guerette, P., Ortlepp, C., & Savage, K. (2002). Elastic proteins: Biological roles and mechanical properties. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 357, 121.
Weis-Fogh, T. (1961). Molecular interpretation of the elasticity of resilin, a rubber-like proteinn. Journal of Molecular Biology, 3, 648.
Elvin, C. M., Carr, A. G., Huson, M. G., Maxwell, J. M., Pearson, R. D., Vuocolo, T., Liyou, N. E., Wong, D. C. C., Merritt, D. J., & Dixon, N. E. (2005). Synthesis and properties of crosslinked recombinant pro-resilin. Nature, 437, 999.
Fudge, D. S., Gardner, K. H., Forsyth, V. T., Riekel, C., & Gosline, J. M. (2003). The mechanical properties of hydrated intermediate filaments: Insights from hagfish slime threads. Biophysical Journal 85, 2015.
Fudge, D. S., & Gosline, J. M. (2004). Molecular design of the alpha-keratin composite: Insights from a matrix-free model, hagfish slime threads. Proceedings of the Royal Society of London Series B-Biological Sciences, 271, 291.
Kreplak, L., Bar, H., Leterrier, J. F., Herrmann, H., & Aebi, U. (2005). Exploring the mechanical behavior of single intermediate filaments. Journal of Molecular Biology, 354, 569.
Mucke, N., Kreplak, L., Kirmse, R., Wedig, T., Herrmann, H., Aebi, U., & Langowski, J. (2004). Assessing the flexibility of intermediate filaments by atomic force microscopy. Journal of Molecular Biology, 335, 1241.
Kreplak, L., & Fudge, D. (2007). Biomechanical properties of intermediate filaments: From tissues to single filaments and back. Bioessays, 29, 26.
Baldock, C., Koster, A. J., Ziese, U., Rock, M. J., Sherratt, M. J., Kadler, K. E., Shuttleworth, C. A., & Kielty, C. M. (2001). The supramolecular organization of fibrillin-rich microfibrils. The Journal of Cell Biology, 152, 1045.
Lu, Y., Holmes, D. F., & Baldock, C. (2005). Evidence for the intramolecular pleating model of fibrillin microfibril organisation from single particle image analysis. Journal of Molecular Biology, 349, 73.
Sherratt, M. J., Baldock, C., Haston, J. L., Holmes, D. F., Jones, C. J. P., Shuttleworth, C. A., Wess, T. J., & Kielty, C. M. (2003). Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. Journal of Molecular Biology, 332, 183.
Megill, W. M., Gosline, J. M., & Blake, R. W. (2005). The modulus of elasticity of fibrillin-containing elastic fibres in the mesoglea of the hydromedusa Pollyorchis penicillatus. Journal of Experimental Biology, 208, 3819.
Wright, D. M., Duance, V. C., Wess, T. J., Kielty, C. M., & Purslow, P. P. (1999). The supramolecular organisation of fibrillin-rich microfibrils determines the mechanical properties of bovine zonular filaments. Journal of Experimental Biology, 202, 3011.
Wang, K., McCarter, R., Wright, J., Beverly, J., & Ramirezmitchell, R. (1993). Viscoelasticity of the sarcomere matrix of skeletal-muscles—the titin myosin composite filament is a dual-stage molecular spring. Biophysical Journal, 64, 1161.
Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M., & Gaub, H. E. (1997). Reversible unfolding of individual titin immunoglobulin domains by AFM. Science, 276, 1109.
Hao, Y. D., Bernstein, S. I., & Pollack, G. H. (2004). Passive stiffness of drosophila IFM myofibrils: A novel, high accuracy measurement method. Journal of Muscle Research and Cell Motility, 25, 359.
Bell, E., & Gosline, J. (1996). Mechanical design of mussel byssus: Material yield enhances attachment strength. The Journal of Experimental Biology, 199, 1005.
Waite, J. H., Qin, X. -X., & Coyne, K. J. (1998). The peculiar collagens of mussel byssus. Matrix Biology, 17, 93.
Waite, J. H., Vaccaro, E., Sun, C. J., & Lucas, J. M. (2002). Elastomeric gradients: A hedge against stress concentration in marine holdfasts? Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 357, 143.
Erickson, H. P. (2002). Stretching fibronectin. Journal of Muscle Research and Cell Motility, 23, 575.
Ohashi, T., Kiehart, D. P., & Erickson, H. P. (2002). Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. Journal of Cell Science, 115, 1221.
Abu-Lail, N. I., Ohashi, T., Clark, R. L., Erickson, H. P., & Zauscher, S. (2006). Understanding the elasticity of fibronectin fibrils: Unfolding strengths of FN-III and GFP domains measured by single molecule force spectroscopy. Matrix Biology, 25, 175.
Baneyx, G., Baugh, L., & Vogel, V. (2002). Supramolecular chemistry and self-assembly special feature: Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. PNAS %R 10.1073/pnas.072650799 99, 5139.
Pins, G. D., Christiansen, D. L., Patel, R., & Silver, F. H. (1997). Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophysical Journal, 73, 2164.
Silver, F. H., Freeman, J. W., & Seehra, G. P. (2003). Collagen self-assembly and the development of tendon mechanical properties. Journal of Biomechanics, 36, 1529.
Silver, F. H., Christiansen, D., Snowhill, P. B., Chen, Y., & Landis, W. J. (2000). The role of mineral in the storage of elastic energy in turkey tendons. Biomacromolecules, 1, 180.
Shadwick, R. E. (1990). Elastic energy-storage in tendons—mechanical differences related to function and age. Journal of Applied Physiology, 68, 1033.
Sasaki, N., & Odajima, S. (1996). Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. Journal of Biomechanics, 29, 655.
Pollock, M., & Shadwick, R. E. (1994). Relationship between body-mass and biomechanical properties of limb tendons in adult mammals. American Journal of Physiology, 266, R1016.
Kojima, H., Ishijima, A., & Yanagida, T. (1994). Direct measurement of stiffness of single actin-filaments with and without tropomyosin by in-vitro nanomanipulation. Proceedings of the National Academy of Sciences of the United States of America, 91, 12962.
Gittes, F., Mickey, B., Nettleton, J., & Howard, J. (1993). Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. The Journal of Cell Biology, 120, 923.
Liu, X., & Pollack, G. H. (2002). Mechanics of F-actin characterized with microfabricated cantilevers. Biophysical Journal, 83, 2705.
Kishino, A., & Yanagida, T. (1988). Force measurements by micromanipulation of a single actin filament by glass needles. Nature, 334, 74.
Kakar, S. K., & Bettelheim, F. A. (1991). Birefringence of actin. Biopolymers, 31, 1283.
Kas, J., Strey, H., Tang, J. X., Finger, D., Ezzell, R., Sackmann, E., & Janmey, P. A. (1996). F-actin, a model polymer for semiflexible chains in dilute, semidilute, and liquid crystalline solutions. Biophysical Journal, 70, 609.
Liu, C. X., Guo, H. L., Xu, C. H., Yuan, M., Li, Z. L., Cheng, B. Y., & Zhang, D. Z. (2005). Measurement of breaking force of fluorescence labelled microtubules with optical tweezers. Chinese Physics Letters, 22, 1278.
Felgner, H., Frank, R., & Schliwa, M. (1996). Flexural rigidity of microtubules measured with the use of optical tweezers. Journal of Cell Science, 109, 509.
Kurachi, M., Hoshi, M., & Tashiro, H. (1995). Buckling of a single microtubule by optical trapping forces—direct measurement of microtubule rigidity. Cell Motility and the Cytoskeleton, 30, 221.
Kis, A., Kasas, S., Babic, B., Kulik, A. J., Benoit, W., Briggs, G. A. D., Schonenberger, C., Catsicas, S., & Forro, L. (2002). Nanomechanics of microtubules. Physical Review Letters, 89, S. 248101.
Wainwright, S. A., Biggs, W. D., Currey, J. D., & Gosline, J. M. (1976). Mechanical design in organisms. Princeton: Princeton University Press.
Hearle, J. W. S. (2000). A critical review of the structural mechanics of wool and hair fibres. International Journal of Biological Macromolecules, 27, 123.
Astbury, W. T., & Street, A. (1931). X-ray studies of the structure of hair, wool and related fibers. I. General Philosophical Transactions of the Royal Society of London, 230A, 75.
Astbury, W. T., & Woods, H. J. (1933). X ray studies of the structure of hair, wool, and related fibres. II. The molecular structure and elastic properties of hair keratin. Philosophical Transactions of the Royal Society of London. Series A: Mathematical and physical Sciences, 232, 333.
Bendit, E. G. (1960). A quantitative X-ray diffraction study of the alpha-beta transformation in wool keratin. Textile Research Journal, 30, 547.
Fraser, R. D., MacRae, T. D., & Rogers, G. E. (1972). Keratins: Their composition, structure, and biosynthesis. Springfield: Charles C. Thomas.
Cohen, C. (1998). Why fibrous proteins are romantic. Journal of Structural Biology, 122, 3.
Strelkov, S. V., Herrmann, H., & Aebi, U. (2003). Molecular architecture of intermediate filaments. Bioessays, 25, 243.
Landau L. D., & Lifschitz, E. M. (1980). Fluctuations in the curvature of long molecules. In Statistical physics part I. Oxford: Pergamon Press.
Guzman, C., Jeney, S., Kreplak, L., Kasas, S., Kulik, A. J., Aebi, U., & Forro, L. (2006). Exploring the mechanical properties of single vimentin intermediate filaments by atomic force microscopy. Journal of Molecular Biology, 360, 623.
Kielty C. M., Sherratt M. J., Marson A., & Baldock, C. (2005). Fibrillin microfibrils. In David A. Parry, & John M. Squire (Eds.) Fibrous proteins: Coiled-coils, collagen and elastomers, 70, 405.
Tskhovrebova, L., & Trinick, J. (2003). Titin: Properties and family relationships. Nature Reviews Molecular Cell Biology, 4, 679.
Sivakumar, P., Czirok, A., Rongish, B. J., Divakara, V. P., Wang, Y. P., & Dallas, S. L. (2006). New insights into extracellular matrix assembly and reorganization from dynamic imaging of extracellular matrix proteins in living osteoblasts. Journal of Cell Science, 119, 1350.
Gao, M., Craig, D., Lequin, O., Campbell, I. D., Vogel, V., & Schulten, K. (2003). Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proceedings of the National Academy of Sciences of the United States of America, 100, 14784.
Bailey, K., Astbury W. T., & Rudall, K. W. (1943). Fibrinogen and fibrin as members of the keratin-myosin group. Nature, 151, 716.
Lorand, L. (1950). Letters to nature. Nature, 166, 694.
Howard, J. (2001). Mechanics of motor proteins and the cytoskeleton. Sunderland: Sinauer Associates.
Roska, F. J., & Ferry, J. D. (1982). Studies of fibrin film. 1. Stress-relaxation and birefringence. Biopolymers, 21, 1811.
Roska, F. J., Ferry, J. D., Lin, J. S., & Anderegg, J. W. (1982). Studies of fibrin film. 2. Small-angle X-ray-scattering. Biopolymers, 21, 1833.
Nelb, G. W., Kamykowski, G. W., & Ferry, J. D. (1981). Rheology of fibrin clots .5. Shear modulus, creep, and creep recovery of fine unligated clots. Biophysical Chemistry, 13, 15.
Gorkun, O. V., Henschen-Edman, A. H., Ping, L. F., & Lord, S. T. (1998). Analysis of A alpha 251 fibrinogen: The alpha C domain has a role in polymerization, albeit more subtle than anticipated from the analogous proteolytic fragment x. Biochemistry, 37, 15434.
Litvinov, R. I., Gorkun, O. V., Owen, S. F., Shuman, H., & Weisel, J. W. (2005). Polymerization of fibrin: Specificity, strength, and stability of knob-hole interactions studied at the single-molecule level. Blood, 106, 2944.
Yang, G., Cecconi, C., Baase, W. A., Vetter, I. R., Breyer, W. A., Haack, J. A., Matthews, B. W., Dahlquist, F. W., & Bustamante, C. (2000). Solid-state synthesis and mechanical unfolding of polymers of T4 lysozyme. Proceedings of the National Academy of Sciences of the United States of America, 97, 139.
Evans, E., & Ritchie, K. (1997). Dynamic strength of molecular adhesion bonds. Biophysical Journal, 72, 1541.
Cohen, I., Gerrard, J. M., & White, J. G. (1982). Ultrastructure of clots during isometric contraction. Journal of Cell Biology, 93, 775.
Acknowledgments
We thank M. C. Stahle, J. L. Moen, and O. V. Gorkun for advice and technical assistance. We acknowledge support from NIH: P41 EB002025 (RS), R01 HL31048 (STL), R41 CA103120 (MG); NSF: CMMI-0646627 (MG); Research Corporation: RI0826 (MG); American Cancer Society: IRG-93-035-6 (MG) and the American Heart Association, Mid-Atlantic Affiliate Grant-in-Aid 055527U (RRH).
Author information
Authors and Affiliations
Corresponding author
Additional information
The senior authors R. R. Hantgan and S. T. Lord have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Guthold, M., Liu, W., Sparks, E.A. et al. A Comparison of the Mechanical and Structural Properties of Fibrin Fibers with Other Protein Fibers. Cell Biochem Biophys 49, 165–181 (2007). https://doi.org/10.1007/s12013-007-9001-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-007-9001-4