Abstract
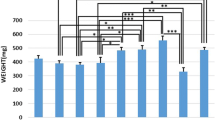
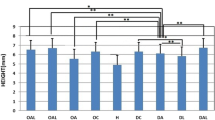
Osteoporosis (OP) and osteoporotic fracture are major public health issues for society; the burden for the affected individual is also high. Previous studies have shown that pulsed wave low-level laser therapy (PW LLLT) has osteogenic effects. This study intended to evaluate the impacts of PW LLLT on the cortical bone of osteoporotic rats’ tibias in two experimental models, ovariectomized and dexamethasone-treated. We divided the rats into four ovariectomized induced OP (OVX-d) and four dexamethasone-treated (glucocorticoid-induced OP, GIOP) groups. A healthy (H) group of rats was considered for baseline evaluations. At 14 weeks following ovariectomy, we subdivided the OVX-d rats into the following groups: (i) control which had OP, (ii) OVX-d rats treated with alendronate (1 mg/kg), (iii) OVX-d rats treated with LLLT, and (iv) OVX-d rats treated with alendronate and PW LLLT. The remaining rats received dexamethasone over a 5-week period and were also subdivided into four groups: (i) control rats treated with intramuscular (i.m.) injections of distilled water (vehicle), (ii) rats treated with subcutaneous alendronate injections (1 mg/kg), (iii) laser-treated rats, and (iv) rats simultaneously treated with laser and alendronate. The rats received alendronate for 30 days and underwent PW LLLT (890 nm, 80 Hz, 0.972 J/cm2) three times per week during 8 weeks. Then, the right tibias were extracted and underwent a stereological analysis of histological parameters and real-time polymerase chain reaction (RT-PCR). A significant increase in cortical bone volume (mm3) existed in all study groups compared to the healthy rats. There were significant decreases in trabecular bone volume (mm3) in all study groups compared to the group of healthy rats. The control rats with OP and rats from the vehicle group showed significantly increased osteoclast numbers compared to most other groups. Alendronate significantly decreased osteoclast numbers in osteoporotic rats. Concurrent treatments (compounded by PW LLLT and alendronate) produce the same effect on osteoporotic bone.









Similar content being viewed by others
Abbreviations
- OP:
-
Osteoporosis
- BMU:
-
Basic multicellular unit
- ECM:
-
Extracellular matrix
- TGF-β:
-
Transforming growth factor beta
- IGF-I:
-
Insulin-like growth factor-I
- BMPs:
-
Bone morphogenetic proteins
- GC:
-
Glucocorticoid
- GIOP:
-
Glucocorticoid-induced osteoporosis
- BMD:
-
Bone mineral density
- LLLT:
-
Low-level laser therapy
- LED:
-
Light-emitting diode of Sohn et al. [15] study
- CW:
-
Continuous wave
- PW:
-
Pulsed wave
- OVX-d:
-
Ovariectomized induced OP
- RT-PCR:
-
Real-time polymerase chain reaction
- cDNA:
-
Complementary DNA
- LSD:
-
Least significant difference
- GTP:
-
Guanosine triphosphate
- AlGaAs:
-
Aluminum gallium arsenide
References
Johnell O, Kanis J (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Syddall HE, Evandrou M, Dennison EM, Cooper C, Sayer AA (2012) Social inequalities in osteoporosis and fracture among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Arch Osteoporos 7(1–2):37–48
Lewiecki EM, Laster AJ (2006) Clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J Clin Endocrinol Metab 91(11):4215–4222
Abrahamsen B, Van Staa T, Ariely R, Olson M, Cooper C (2009) Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 20(10):1633–1650
Riggs BL, Khosla S, Melton LJ 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23(3):279–302
Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R (1998) Does microdamage accumulation affect the mechanical properties of bone? J Biomech 31(4):337–345
Dai R-C, Liao E-Y, Yang C, Wu X-P, Jiang Y (2004) Microcracks: an alternative index for evaluating bone biomechanical quality. J Bone Miner Metab 22(3):215–223
Braidman IP, Hainey L, Batra G, Selby PL, Saunders PT, Hoyland JA (2001) Localization of estrogen receptor β protein expression in adult human bone. J Bone Miner Res 16(2):214–220
Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF (1996) Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med 2(10):1132–1136
McNamara L (2010) Perspective on post-menopausal osteoporosis: establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. J R Soc Interface 7(44):353–372
Khosla S, Westendorf JJ, Oursler MJ (2008) Building bone to reverse osteoporosis and repair fractures. J Clin Invest 118(2):421–428
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg 22(2):141–150
van Brussel MS, Bultink IE, Lems WF (2009) Prevention of glucocorticoid-induced osteoporosis. Expert Opin Pharmacother 10(6):997–1005
Cashman KD (2007) Diet, nutrition, and bone health. J Nutr 137(11):2507S–2512S
Sohn H, Ko Y, Park M, Kim D, Moon YL, Jeong YJ, Lee H, Moon Y, Jeong BC, Kim O, Lim W (2015) Effects of light-emitting diode irradiation on RANKL-induced osteoclastogenesis. Lasers Surg Med 22(10):22413
Kiyosaki T, Mitsui N, Suzuki N, Shimizu N (2010) Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed Laser Surg 28(1)
Shimizu N, Mayahara K, Kiyosaki T, Yamaguchi A, Ozawa Y, Abiko Y (2007) Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg Med 39(6):551–559
Ko CY, Kang H, Seo DH, Jung B, Schreiber J, Kim HS (2013) Low-level laser therapy using the minimally invasive laser needle system on osteoporotic bone in ovariectomized mice. Med Eng Phys 35(7):1015–1019
Kang H, Ko CY, Ryu Y, Seo DH, Kim HS, Jung B (2012) Development of a minimally invasive laser needle system: effects on cortical bone of osteoporotic mice. Lasers Med Sci 27(5):965–969
Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) Effects of 830-nm laser light on preventing bone loss after ovariectomy. Photomed Laser Surg 24(5):642–645
Muniz Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) The effects of infrared-830 nm laser on exercised osteopenic rats. Lasers Med Sci 21(4):202–207
Medalha CC, Amorim BO, Ferreira JM, Oliveira P, Pereira RM, Tim C, Lirani-Galvao AP, da Silva OL, Renno AC (2010) Comparison of the effects of electrical field stimulation and low-level laser therapy on bone loss in spinal cord-injured rats. Photomed Laser Surg 28(5):669–674
Fridoni M, Masteri Farahani R, Nejati H, Salimi M, Gharavi SM, Bayat M, Amini A, Torkman G, Bayat S (2015) Evaluation of the effects of LLLT on biomechanical properties of tibial diaphysis in two rat models of experimental osteoporosis by a three point bending test. Lasers Med Sci 30(3):1117–1125
Kanis J, McCloskey E, Johansson H, Cooper C, Rizzoli R, Reginster J-Y (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24(1):23–57
Li X, Ominsky MS, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Dwyer D, Grisanti M, Stolina M, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ (2011) Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats. Endocrinology 152(9):3312–3322
Ferretti JL, Gaffuri O, Capozza R, Cointry G, Bozzini C, Olivera M, Zanchetta JR, Bozzini CE (1995) Dexamethasone effects on mechanical, geometric and densitometric properties of rat femur diaphyses as described by peripheral quantitative computerized tomography and bending tests. Bone 16(1):119–124
Sun P, Cai DH, Li QN, Chen H, Deng WM, He L, Yang L (2010) Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int 86(6):495–501
Sterio D (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134(2):127–136
Gundersen HJ, Jensen EB, Kieu K, Nielsen J (1999) The efficiency of systematic sampling in stereology—reconsidered. J Microsc 193(Pt 3):199–211
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A et al (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96(5):379–394
Gundersen H-JG (1986) Stereology of arbitrary particles. J Microsc 143(1):3–45
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25(4):402–408
Yeh JK, Chen MM, Aloia JF (1996) Ovariectomy-induced high turnover in cortical bone is dependent on pituitary hormone in rats. Bone 18(5):443–450
Jagtap VR, Ganu JV, Nagane NS (2011) BMD and serum intact osteocalcin in postmenopausal osteoporosis women. Indian J Clin Biochem 26(1):70–73
Green D, Wallace H (2003) Late effects of childhood cancer. CRC Press
Parfitt AM (1987) Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med 82(1B):68–72
Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF (2006) Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res 21(3):466–476
Chao EY, Inoue N, Koo TK, Kim YH (2004) Biomechanical considerations of fracture treatment and bone quality maintenance in elderly patients and patients with osteoporosis. Clin Orthop Relat Res 425:12–25
Roschger P, Rinnerthaler S, Yates J, Rodan G, Fratzl P, Klaushofer K (2001) Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 29(2):185–191
Oei L, Zillikens MC, Rivadeneira F, Oei EH (2015) Osteoporotic vertebral fractures as part of systemic disease. J Clin Densitom
Russell RG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119(2):S150–S162
Diniz JS, Nicolau RA, de Melo Ocarino N, do Carmo Magalhaes F, de Oliveira Pereira RD, Serakides R (2009) Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci 24(3):347–352
Pires-Oliveira DA, Oliveira RF, Amadei SU, Pacheco-Soares C, Rocha RF (2010) Laser 904 nm action on bone repair in rats with osteoporosis. Osteoporos Int 21(12):2109–2114
Re Poppi R, Da Silva AL, Nacer RS, Vieira RP, de Oliveira LV, Santos de Faria Junior N, de Tarso Camilo Carvalho P (2011) Evaluation of the osteogenic effect of low-level laser therapy (808 nm and 660 nm) on bone defects induced in the femurs of female rats submitted to ovariectomy. Lasers Med Sci 26(4):515–522
Scalize PH, de Sousa LG, Regalo SC, Semprini M, Pitol DL, da Silva GA, de Almeida CJ, Coppi AA, Laad AA, Prado KF, Siessere S (2015) Low-level laser therapy improves bone formation: stereology findings for osteoporosis in rat model. Lasers Med Sci 30(5):1599–1607
Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Peitl O, Zanotto ED, Parizotto NA (2011) Biosilicate® and low-level laser therapy improve bone repair in osteoporotic rats. J Tissue Eng Regen Med 5(3):229–237
Fangel R, Bossini PS, Renno AC, Ribeiro DA, Wang CC, Toma RL, Nonaka KO, Driusso P, Parizotto NA, Oishi J (2011) Low-level laser therapy, at 60 J/cm2 associated with a Biosilicate(®) increase in bone deposition and indentation biomechanical properties of callus in osteopenic rats. J Biomed Opt 16(7):3598847
Fangel R, Bossini PS, Renno AC, Granito RN, Wang CC, Nonaka KO, Driusso P, Parizotto NA, Oishi J (2014) Biomechanical properties: effects of low-level laser therapy and Biosilicate® on tibial bone defects in osteopenic rats. J Appl Biomater Funct Mater 12(3):271–277
Freidouni M, Nejati H, Salimi M, Bayat M, Amini A, Noruzian M, Asgharie MA, Rezaian M (2015) Evaluating glucocorticoid administration on biomechanical properties of rats’ tibial diaphysis. Iran Red Crescent Med J 17(3)
Chow RT (2000) Dose dilemmas in low level laser therapy-the effects of different paradigms and historical perspectives. Laser Therapy 13(1):102–109
Bayat M, Ansari E, Gholami N, Bayat A (2007) Effect of low-level helium–neon laser therapy on histological and ultrastructural features of immobilized rabbit articular cartilage. J Photochem Photobiol B Biol 87(2):81–87
Acknowledgments
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei. This article was financially supported by the Research Department of the School of Medicine and the Vice Chancellor of Research at Shahid Beheshti University of Medical Sciences, Tehran, Iran (grants: 1393-1-91-1350 and 1392-1-115-1160).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal rights and informed consent
All procedure were approved by the Medical Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, Iran (Protocols no.:1393-1-91-1350 and 1392-1-115-1160).
Rights and permissions
About this article
Cite this article
Mohsenifar, Z., Fridoni, M., Ghatrehsamani, M. et al. Evaluation of the effects of pulsed wave LLLT on tibial diaphysis in two rat models of experimental osteoporosis, as examined by stereological and real-time PCR gene expression analyses. Lasers Med Sci 31, 721–732 (2016). https://doi.org/10.1007/s10103-016-1916-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-1916-9