Abstract
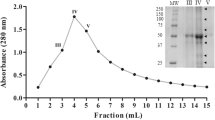
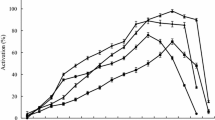
The latex from Vasconcellea quercifolia (“oak leaved papaya”), a member of the Caricaceae family, contains at least seven cysteine endopeptidases with high proteolytic activity, which helps to protect these plants against injury. In this study, we isolated and characterized the most basic of these cysteine endopeptidases, named VQ-VII. This new purified enzyme was homogeneous by bidimensional electrophoresis and MALDI-TOF mass spectrometry, and exhibited a molecular mass of 23,984 Da and an isoelectric point >11. The enzymatic activity of VQ-VII was completely inhibited by E-64 and iodoacetic acid, confirming that it belongs to the catalytic group of cysteine endopeptidases. By investigating the cleavage of the oxidized insulin B-chain to establish the hydrolytic specificity of VQ-VII, we found 13 cleavage sites on the substrate, revealing that it is a broad-specificity peptidase. The pH profiles toward p-Glu-Phe-Leu-p-nitroanilide (PFLNA) and casein showed that the optimum pH is about 6.8 for both substrates, and that in casein, it is active over a wide pH range (activity higher than 80 % between pH 6 and 9.5). Kinetic enzymatic assays were performed with the thiol peptidase substrate PFLNA (K m = 0.454 ± 0.046 mM, k cat = 1.57 ± 0.07 s−1, k cat/K m = 3.46 × 103 ± 14 s−1 M−1). The N-terminal sequence (21 amino acids) of VQ-VII showed an identity >70 % with 11 plant cysteine peptidases and the presence of highly conserved residues and motifs shared with the “papain-like” family of peptidases. VQ-VII proved to be a new latex enzyme of broad specificity, which can degrade extensively proteins of different nature in a wide pH range.




Similar content being viewed by others
Abbreviations
- AMPSO:
-
3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxy-propanesulfonic acid
- BLAST:
-
Basic local alignment search tool
- CAPS:
-
3-(Cyclohexylamino)-1-propanesulfonic acid
- CHAPS:
-
3-[(3-Cholamidopropyl)-dimethylammonio]-1-propane sulfonate
- 2D-PAGE:
-
Two dimensional-polyacrylamide gel electrophoresis
- DTT:
-
Dithiothreitol
- E-64:
-
Trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane
- HCCA:
-
α-Cyano-4-hydroxy-cinnamic acid
- MALDI-TOF MS:
-
Matrix-assisted laser desorption/ionization time of flight mass spectrometry
- MES:
-
2-Morpholinoethanesulfonic acid
- MOPS:
-
3-(N-morpholino) propanesulfonic acid
- PFLNA:
-
p-Glu-Phe-Leu-p-nitroanilide
- PMF:
-
Peptide mass fingerprinting
- SDS:
-
Sodium dodecyl sulfate
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SP:
-
Sulfopropyl
- TAPS:
-
N-tris (hydroxymethyl) methyl-3-aminopropanesulfonic acid
- TFA:
-
Trifluoroacetic acid
References
Abreu Payrol J, Obregón WD, Trejo SA, Caffini NO (2008) Purification and characterization of four new cysteine endopeptidases from fruits of Bromelia pinguin L. grown in Cuba. Protein J 27:88–96
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Barrett AJ, Kirschke H (1981) Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol 80:535–561
Barrett AJ, Rawlings ND (2004) Introduction: the clans and families of cysteine peptidases. In: Barrett AJ, Rawlings ND, Woessner JF (eds) Handbook of proteolytic enzymes, 2nd edn. Elsevier Academic Press, London, pp 1051–1071
Bradford MM (1976) A rapid and sensitive method for the quantitation of micrograms quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bruno MA, Pardo MA, Caffini NO, López LMI (2003) Hieronymain I, a new cysteine peptidase isolated from unripe fruits of Bromelia hieronymi Mez (Bromeliaceae). J Protein Chem 22:127–134
Bruno MA, Trejo SA, Avilés XF, Caffini NO, López LMI (2006) Isolation and characterization of Hieronymain II, another peptidase isolated from fruits of Bromelia hieronymi Mez (Bromeliaceae). Protein J 25:224–231
Bruno MA, Trejo SA, Caffini NO, López LMI (2008) Purification and characterization of hieronymain III. Comparison with other proteases previously isolated from Bromelia hieronymi Mez. Protein J 27:426–433
Deguchi Y, Banba M, Shimoda Y, Chechetka SA, Suzuri R, Okusako Y, Ooki Y, Toyokura K, Suzuki A, Uchiumi T, Higashi S, Abe M, Kouchi H, Izui K, Hata S (2007) Transcriptome profiling of Lotus japonicus roots during arbuscular mycorrhizal development and comparison with that of nodulation. DNA Res 14:117–133
Domsalla A, Melzig MF (2008) Occurrence and properties of proteases in plant latices. Planta Med 74:699–711
Filippova IY, Lysogorskaya EN, Oksenoit ES, Rudenskaya GN, Stepanov VM (1984) l-Pyroglutamyl-l-phenylalanyl-l-leucine-p-nitroanilide—a chromogenic substrate for thiol proteinase assay. Anal Biochem 143:293–297
Gomes MTR, Teixeira RD, Ribeiro HA, Turchetti AP, Junqueira CF, Lopes MTP, Salas CE, Nagem RA (2008) Purification, crystallization and preliminary X-ray analysis of CMS1MS2: a cysteine proteinase from Carica candamarcensis latex. Acta Crystallogr F 64:492–494
Gomes MTR, Ribeiro HA, Lopes MTP, Guzman F, Salas CE (2010) Biochemical comparison of two proteolytic enzymes from Carica candamarcensis: structural motifs underlying resistance to cystatin inhibition. Phytochemistry 71:524–530
Good NE, Izawa S (1972) Hydrogen ion buffers. Methods Enzymol 24:53–68
Hames BD (1990) One-dimensional polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of proteins, 2nd edn. Oxford University Press, Oxford, pp 1–147
Kaneda M, Nagatome S, Uchikoba T (1995) Comparison of phytolacain R, a cysteine protease from Phytolacca americana, with papain. Phytochemistry 39:997–999
Konno K, Hirayama C, Nakamura M, Tateishi K, Tamura Y, Hattori M, Kohno K (2004) Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J 37:370–378
Kyndt T, Van Damme EJ, Van Beeumen J, Gheysen G (2007) Purification and characterization of the cysteine proteinases in the latex of Vasconcellea spp. FEBS J 274:451–462
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
López LMI, Sequeiros C, Natalucci CL, Brullo A, Maras B, Barra D, Caffini NO (2000) Purification and characterization of macrodantin I, a cysteine proteinase from unripe fruits of Pseudananas macrodontes (Morr.) Harms (Bromeliaceae). Protein Express Purif 18:133–140
Martínez M, Díaz I (2008) The origin and evolution of plant cystatins and their target cysteine proteinases indicate a complex functional relationship. BMC Evol Biol 8:198–209
Martínez M, Cambra I, Gonzalez-Melendi P, Santamaría ME, Díaz I (2012) C1A cysteine-proteases and their inhibitors in plants. Physiol Plant 145:85–94
McLellan H, Gilroy EM, Yun BW, Birch PR, Loake GJ (2009) Functional redundancy in the Arabidopsis cathepsin B gene family contributes to basal defence, the hypersensitive response and senescence. New Phytol 183:408–418
Mitsuhashi W, Yamashita T, Toyomasu T, Kashiwagi Y, Konnai T (2004) Sequential development of cysteine proteinase activities and gene expression during somatic embryogenesis in carrot. Biosci Biotechnol Biochem 68:705–713
Morcelle SR, Trejo SA, Canals F, Avilés FX, Priolo NS (2004) Funastrain c II: a cysteine endopeptidase purified from the latex of Funastrum clausum. Protein J 23:205–215
Nadeau JA, Zhang XS, Li J, O’Neill SD (1996) Ovule development: identification of stage-specific and tissue-specific cDNAs. Plant Cell 8:213–239
Natalucci CL, Brullo A, López LM, Hilal RM, Caffini NO (1996) Macrodantin, a new protease isolated from fruits of Pseudananas macrodontes (Morr.) Harms (Bromeliaceae). J Food Biochem 19:443–454
Obregón WD, Lufrano D, Liggieri CS, Trejo SA, Vairo Cavalli SE, Avilés FX, Priolo NS (2011) Biochemical characterization, cDNA cloning, and molecular modeling of araujiain aII, a papain-like cysteine protease from Araujia angustifolia latex. Planta 234:293–304
Pérez A, Carvajal C, Trejo S, Torres MJ, Martin MI, Lorenzo JC, Natalucci CL, Hernández M (2010) Penduliflorain I: a cysteine protease isolated from Hohenbergia penduliflora (A.Rich.) Mez (Bromeliaceae). Protein J 29:225–233
Ramos-Martínez EM, Herrera-Ramírez AC, Badillo-Corona JA, Garibay-Orijel C, González-Rabade N, Oliver-Salvador MDC (2012) Isolation of cDNA from Jacaratia mexicana encoding a mexicain-like cysteine protease gene. Gene 502:60–68
Rawlings ND, Barrett AJ, Bateman A (2012) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 40D1:D343–D350
Salvesen G, Nagase H (1989) Inhibition of proteolytic enzymes. In: Beynon RJ, Bond JS (eds) Proteolytic enzymes, a practical approach. IRL Press, Oxford, pp 83–104
Scheldeman X, Willemen L, Coppens D’Eeckenbrugge G, Romeijn-Peeters E, Restrepo MT, Romero Motoche J, Jimenez DR, Lobo M, Medina CI, Reyes C, Rodriguez D, Ocampo Perez JA, Van Damme P, Goetgebeur P (2007) Distribution, diversity and environmental adaptation of highland papayas (Vasconcellea spp.) in tropical and subtropical America. Biodivers Conserv 16:1867–1884
Sequeiros C, Torres MJ, Trejo SA, Esteves JL, Natalucci CL, López LMI (2005) Philibertain g I, the most basic cysteine endopeptidase purified from the latex of Philibertia gilliesii Hook. et Arn. (Apocynaceae). Protein J 24:445–453
Shindo T, Van der Hoorn RAL (2008) Papain-like cysteine proteases: key players at molecular battlefields employed by both plants and their invaders. Mol Plant Pathol 9:119–125
Siigur J, Trummal K, Tônismägi K, Samel M, Siigur E, Vija H, Tammiste I, Subbi J (2002) Use of MALDI-TOF mass spectrometry for specificity studies of biomedically important proteases. Spectroscopy 16:103–409
Thomas MP, Verma C, Boyd SM, Brocklehurst K (1995) The structural origins of the unusual specificities observed in the isolation of chymopapain M and actinidin by covalent chromatography and the lack of inhibition of chymopapain M by cystatin. Biochem J 306:39–46
Torres MJ, Trejo SA, Martin MI, Natalucci CL, Avilés FX, López LMI (2010) Purification and characterization of a cysteine endopeptidase from Vasconcellea quercifolia A. St.-Hil. latex displaying high substrate specificity. J Agric Food Chem 58:11027–11035
Torres MJ, Trejo SA, Obregón WD, Avilés FX, López LMI, Natalucci CL (2012) Characterization of the proteolytic system present in Vasconcellea quercifolia latex. Planta 236:1471–1484
Trejo SA (2005) Purificación, caracterización bioquímica y estructural y expresión de una endopeptidasas cisteínicas de látex de Asclepias fruticosa L. (Apocynaceae). PhD Thesis, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Argentina
Trejo SA, López LMI, Caffini NO, Natalucci CL, Canals F, Avilés FX (2009) Sequencing and characterization of asclepain f: the first cysteine peptidase cDNA cloned and expressed from Asclepias fruticosa latex. Planta 230:319–328
Turk D, Guncar G, Podobnik M, Turk B (1998) Revised definition of substrate binding sites of papain-like cysteine proteases. Biol Chem 379:137–147
Vallés D, Bruno M, López LMI, Caffini NO, Cantera AMB (2008) Granulosain I, a cysteine protease isolated from ripe fruits of Solanum granuloso-leprosum (Solanaceae). Protein J 27:267–275
van der Hoorn RA (2008) Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol 59:191–223
Walreavens V, Jaziri M, van Beeumen J, Schnek AG, Kleinschmidt T, Looze Y (1993) Isolation and preliminary characterization of the cysteine-proteinases from the latex of Carica candamarcensis Hook. Biol Chem Hoppe-Seyler 374:501–506
Acknowledgments
The present work was supported by grants from CONICET (PIP 0477), UNLP and CIC (Argentina). C.L. Natalucci is member of the CICPBA Researcher Career, L.M.I. López is member of the CONICET Researcher Career. The MALDI-TOF MS experiments were carried out in the proteomics facility (SePBioEs) from IBB-UAB, a member of ProteoRed-ISCIII.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. L. Natalucci and L. M. I. López contributed equally to this work.
Rights and permissions
About this article
Cite this article
Torres, M.J., Trejo, S.A., Natalucci, C.L. et al. Biochemical characterization of VQ-VII, a cysteine peptidase with broad specificity, isolated from Vasconcellea quercifolia latex. Planta 237, 1651–1659 (2013). https://doi.org/10.1007/s00425-013-1872-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-1872-6