Abstract
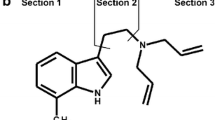
N,N-Diallyltryptamine (DALT) and 5-methoxy-DALT (5-MeO-DALT) are synthetic tryptamine derivatives commonly referred to as so-called new psychoactive substances (NPS). They have psychoactive effects that may be similar to those of other tryptamine derivatives. The objectives of this work were to study the metabolic fate and detectability, in urine, of DALT and 5-MeO-DALT. For metabolism studies, rat urine obtained after high-dose administration was prepared by precipitation and analyzed by liquid chromatography–high-resolution mass spectrometry (LC–HR–MS–MS). On the basis of the metabolites identified, several aromatic and aliphatic hydroxylations, N-dealkylation, N-oxidation, and combinations thereof are proposed as the main metabolic pathways for both compounds. O-Demethylation of 5-MeO-DALT was also observed, in addition to extensive glucuronidation or sulfation of both compounds after phase I transformation. The cytochrome P450 (CYP) isoenzymes predominantly involved in DALT metabolism were CYP2C19, CYP2D6, and CYP3A4; those mainly involved in 5-MeO-DALT metabolism were CYP1A2, CYP2C19, CYP2D6, and CYP3A4. For detectability studies, rat urine was screened by GC–MS, LC–MSn, and LC–HR–MS–MS after administration of low doses. LC–MSn and LC–HR–MS–MS were deemed suitable for monitoring consumption of both compounds. The most abundant targets were a ring hydroxy metabolite of DALT, the N,O-bis-dealkyl metabolite of 5-MeO-DALT, and their glucuronides. GC–MS enabled screening of DALT by use of its main metabolites only.




Similar content being viewed by others
References
United Nations Office on Drugs and Crime (UNODC) (2014) World Drug Report 2014. http://www.unodc.org/documents/data-and-analysis/WDR2014/World_Drug_Report_2014_web.pdf
Brandt SD, King LA, Evans-Brown M (2014) The new drug phenomenon. Drug Test Anal 6:587–597
Meyer MR, Caspar A, Brandt SD, Maurer HH (2014) A qualitative/quantitative approach for the detection of 37 tryptamine-derived designer drugs, 5 beta-carbolines, ibogaine, and yohimbine in human urine and plasma using standard urine screening and multi-analyte approaches. Anal Bioanal Chem 406:225–237
Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (2014) Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology (Berl) 231:4135–4144
Cozzi NV, Gopalakrishnan A, Anderson LL, Feih JT, Shulgin AT, Daley PF, Ruoho AE (2009) Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural Transm 116:1591–1599
Halberstadt AL, Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61:364–381
Halberstadt AL, Koedood L, Powell SB, Geyer MA (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol 25:1548–1561
Nichols DE (2012) Structure-activity relationships of serotonin 5-HT2A agonists. WIREs Membr Transport Signaling 1:559–579
Shulgin AT (2003) Basic pharmacology and effects. In: Laing R R, Halluinogens A Forensic drug handbook. Elsevier Science, London, 67–137
Shulgin AT, Shulgin A (2004) 5-MeO-DALT. http://isomerdesign.com/PiHKAL/read.php?domain=tk&id=56
Shulgin AT, Shulgin A (2004) DALT. http://isomerdesign.com/PiHKAL/read.php?domain=tk&id=57
Corkery JM, Durkin E, Elliott S, Schifano F, Ghodse AH (2012) The recreational tryptamine 5-MeO-DALT (N, N-diallyl-5-methoxytryptamine): a brief review. Prog Neuropsychopharmacol Biol Psychiatry 39:259–262
Jovel A, Felthous A, Bhattacharyya A (2014) Delirium due to intoxication from the novel synthetic tryptamine 5-MeO-DALT. J Forensic Sci 59:844–846
Brandt SD, Martins CPB (2010) Analytical methods for psychoactive N, N-dialkylated tryptamines. Trends Anal Chem 29:858–869
Gaujac A, Navickiene S, Collins MI, Brandt SD, de Andrade JB (2012) Analytical techniques for the determination of tryptamines and beta-carbolines in plant matrices and in psychoactive beverages consumed during religious ceremonies and neo-shamanic urban practices. Drug Test Anal 4:636–648
Katagi M, Kamata T, Zaitsu K, Shima N, Kamata H, Nakanishi K, Nishioka H, Miki A, Tsuchihashi H (2010) Metabolism and toxicologic analysis of tryptamine-derived drugs of abuse. Ther Drug Monit 32:328–331
Brandt SD, Tirunarayanapuram SS, Freeman S, Dempster N, Barker SA, Daley PF, Cozzi NV, Martins CPB (2008) Microwave-accelerated synthesis of psychoactive deuterated N, N-dialkylated-[alpha, alpha, beta, beta-d(4)]-tryptamines. J Label Compd Radiopharm 51:423–429
Wissenbach DK, Meyer MR, Remane D, Weber AA, Maurer HH (2011) Development of the first metabolite-based LC–MSn urine drug screening procedure - exemplified for antidepressants. Anal Bioanal Chem 400:79–88
Meyer MR, Vollmar C, Schwaninger AE, Maurer HH (2012) New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: studies on their metabolism in rat urine and human liver microsomes using GC–MS and LC-high-resolution MS and their detectability in urine. J Mass Spectrom 47:253–262
Helfer AG, Turcant A, Boels D, Ferec S, Lelievre B, Welter J, Meyer MR, Maurer HH (2015) Elucidation of the metabolites of the novel psychoactive substance 4-methyl-N-ethyl-cathinone (4-MEC) in human urine and pooled liver microsomes by GC–MS and LC–HR–MS–MS techniques and of its detectability by GC–MS or LC–MSn standard screening approaches. Drug Test Anal 7:368–375
Maurer HH, Pfleger K, Weber AA (2011) Mass spectral and GC data of drugs, poisons, pesticides, pollutants and their metabolites. Wiley-VCH, Weinheim (Germany)
Welter J, Kavanagh P, Maurer HH (2014) GC–MS and LC-(high-resolution)-MSn studies on the metabolic fate and detectability of camfetamine in rat urine. Anal Bioanal Chem 406:3815–3829
Maurer HH, Pfleger K, Weber AA (2015) Mass spectral library of drugs, poisons, pesticides, pollutants and their metabolites. Wiley-VCH, Weinheim
Meyer MR, Peters FT, Maurer HH (2010) Automated mass spectral deconvolution and identification system for GC–MS screening for drugs, poisons, and metabolites in urine. Clin Chem 56:575–584
Maurer HH, Wissenbach DK, Weber AA (2014) Maurer/Wissenbach/Weber MWW LC–MSn Library of drugs, poisons, and their metabolites. Wiley-VCH, Weinheim
Niessen WMA (2011) Fragmentation of toxicologically relevant drugs in positive-ion liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev 30:626–663
Caspar AT, Helfer AG, Michely JA, Auwaerter V, Brandt SD, Meyer MR, Maurer HH (2015) Studies on the metabolism and toxicological detection of the new psychoactive designer drug 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe) in human and rat urine using GC–MS, LC–MSn, and LC–HR–MS–MS. Anal Bioanal Chem. doi:10.1007/s00216-015-8828-6
Welter J, Kavanagh P, Meyer MR, Maurer HH (2015) Benzofuran analogues of amphetamine and methamphetamine: studies on the metabolism and toxicological analysis of 5-APB and 5-MAPB in urine and plasma using GC–MS and LC-(HR)-MSn techniques. Anal Bioanal Chem 407:1371–1388
Wink CSD, Meyer MR, Braun T, Turcant A, Maurer HH (2015) Biotransformation and detectability of the designer drug 2,5-dimethoxy-4-propylphenethylamine (2C-P) studied in urine by GC–MS, LC–MSn and LC-high resolution-MSn. Anal Bioanal Chem 407:831–843
Meyer MR, Mauer S, Meyer GMJ, Dinger J, Klein B, Westphal F, Maurer HH (2014) The in vivo and in vitro metabolism and the detectability in urine of 3',4'-methylenedioxy-alpha-pyrrolidinobutyrophenone (MDPBP), a new pyrrolidinophenone-type designer drug, studied by GC–MS and LC–MSn. Drug Test Anal 6:746–756
Welter J, Meyer MR, Wolf E, Weinmann W, Kavanagh P, Maurer HH (2013) 2-Methiopropamine, a thiophene analogue of methamphetamine: studies on its metabolism and detectability in the rat and human using GC–MS and LC-(HR)-MS techniques. Anal Bioanal Chem 405:3125–3135
Meyer MR, Wilhelm J, Peters FT, Maurer HH (2010) Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem 397:1225–1233
Philipp AA, Wissenbach DK, Weber AA, Zapp J, Zoerntlein SW, Klein ON, Kanogsunthornrat J, Maurer HH (2010) Use of liquid chromatography coupled to low- and high-resolution linear ion trap mass spectrometry for studying the metabolism of paynantheine, an alkaloid of the herbal drug Kratom in rat and human urine. Anal Bioanal Chem 396:2379–2391
Philipp AA, Wissenbach DK, Weber AA, Zapp J, Maurer HH (2010) Phase I and II metabolites of speciogynine, a diastereomer of the main Kratom alkaloid mitragynine, identified in rat and human urine by liquid chromatography coupled to low and high resolution linear ion trap mass spectrometry. J Mass Spectrom 45:1344–1357
Sharma V, McNeill JH (2009) To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157:907–921
Kovats E (1958) Gaschromatographische Charakterisierung organischer Verbindungen. Teil 1. Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv Chim Acta 41:1915–1932
Acknowledgments
The authors thank Achim T. Caspar, Julia Dinger, Golo M. J. Meyer, Jessica Welter, Carina S. D. Wink, Carsten Schröder, Gabriele Ulrich, and Armin A. Weber for their support and/or helpful discussion.
Conflict of interest
The authors declare there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 4945 kb)
Rights and permissions
About this article
Cite this article
Michely, J.A., Helfer, A.G., Brandt, S.D. et al. Metabolism of the new psychoactive substances N,N-diallyltryptamine (DALT) and 5-methoxy-DALT and their detectability in urine by GC–MS, LC–MSn, and LC–HR–MS–MS. Anal Bioanal Chem 407, 7831–7842 (2015). https://doi.org/10.1007/s00216-015-8955-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8955-0