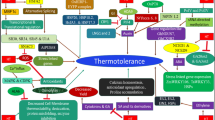
Abstract
Of the various environmental stresses that a plant can experience, temperature has the widest and most far-reaching effects on legumes. Temperature extremes, both high (heat stress) and low (cold stress), are injurious to plants at all stages of development, resulting in severe loss of productivity. In response to unfavorable temperatures, plant biomolecules such as stress proteins, enzymatic and non-enzymatic antioxidants, organic osmolytes and phytohormones come into play, usually, as a part of the plant defense mechanisms. The accumulation of these molecules, which may be useful as metabolic indicators of stress tolerance, depend on the plant species exposed to the temperature stress, its intensity and duration. Some of these molecules such as osmolytes, non-enzymatic antioxidants and phytohormones may be supplied exogenously to improve temperature stress tolerance. Legumes show varying degrees of sensitivity to high and low-temperature stresses, which reduces their potential performance at various developmental stages. To address the ever-fluctuating temperature extremes that various legumes are being constantly exposed, efforts are being made to develop tolerant plant varieties via conventional breeding methods as well as more recent molecular breeding techniques. In this review, we describe the progress made towards the adverse effects of abnormal temperatures on various growth stages in legumes and propose appropriate strategies to resolve these effects.

Source: FAOSTAT; http://faostat3.fao.org

Source: FAOSTAT; http://faostat3.fao.org



Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- APO:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- ASA:
-
Acetyl salicylic acid
- BAC:
-
Bacterial artificial chromosome
- BR:
-
Brassinosteroid
- cADPR:
-
Cyclic ADP-ribose
- CAT:
-
Catalase
- CIAT:
-
International Center for Tropical Agriculture
- cos :
-
Cold suppressed gene
- CSP:
-
Cold-shock proteins
- FAOSTAT:
-
Food and Agriculture Organization Statistics
- GB:
-
Glycine betaine
- GR:
-
Glutathione reductase
- GroEL:
-
A molecular chaperone
- GSH:
-
Glutathione
- HSP:
-
Heat-shock proteins
- ICARDA:
-
International Center for Agricultural Research in the Dry Areas
- ICRISAT:
-
International Crops Research Institute for the Semi-Arid Tropics
- IP3 :
-
Inositol triphosphate
- NO:
-
Nitric oxide
- OEC:
-
Oxygen evolving complex
- O–J–I–P:
-
Transient fluorescence induction transient defined by the names of its intermediate steps
- PAs:
-
Polyamines
- POX:
-
Peroxidase
- RCA:
-
Ribulose activase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SNP:
-
Sodium nitroprusside
- SOD:
-
Superoxide dismutase
References
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory function in plant abiotic stress tolerance. Planta 231:1237–1249
Alexandre A, Oliveira S (2011) Most heat-tolerant rhizobia show high induction of major chaperone genes upon stress. FEMS Microbiol Ecol 75:28–36
Alexandre A, Oliveira S (2013) Response to temperature stress in rhizobia. Crit Rev Microbiol 39:219–228
Ali A, Johnson DL, Stushnoff C (1999) Screening lentil (Lens culinaris) for cold hardiness under controlled conditions. J Agr Sci 133:313–319
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6:36–42
Arbaoui M, Balko C, Link W (2008) Study of faba bean (Vicia faba L.) winter-hardiness and development of screening methods. Field Crop Res 106:60–67
Aro EM, Hundal T, Carlberg I, Andersson B (1990) In vitro studies on light-induced inhibition of Photosystem II and D1-protein degradation at low temperatures. Biochim Biophys Acta 1019:269–275
Awasthi R, Bhandari K, Nayyar H (2015) Temperature stress and redox homeostasis in agricultural crops. Front Environ Sci 3:11–35
Badaruddin M, Meyer DW (2001) Factors modifying frost tolerance of legume species. Crop Sci 41:1911–1916
Bakht J, Bano A, Dominy P (2006) The role of abscisic acid and low temperature in chickpea (Cicer arietinum) cold tolerance. II. Effects on plasma membrane structure and function. J Exp Bot 57:3707–3715
Barghi SS, Mostafaii H, Peighami F, Zakaria RA (2012) Path analysis of yield and its components in lentil under end season heat condition. Int J Agric Res Rev 2:969–974
Barghi SS, Mostafaii H, Peighami F, Zakaria RA, Nejhad RF (2013) Response of in vitro pollen germination and cell membrane thermostability of lentil genotypes to high temperature. Int J Agric Res Rev 3:13–20
Bhandari K, Nayyar H (2014) Low temperature stress in plants: an overview of roles of cryoprotectants in defense. In: Ahmed P, Wani MdR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment, 1st edn. Springer, New York, pp 193–265
Board JE, Kahlon CS (2011) Soybean yield formation: what controls it and how it can be improved? In: El-Shemy HA (ed) Soybean physiology and biochemistry. InTech Open Access, Rijeka, pp 1–36
Boote KJ, Allen LH, Prasad PVV, Baker JT, Gesch RW, Snyder AM, Pan D, Thomas JMG (2005) Elevated temperature and CO2 impacts on pollination, reproductive growth, and yield of several globally important crops. J Agric Meteorol 60:469–474
Canci H, Toker C (2009) Evaluation of yield criteria for drought and heat resistance in chickpea (Cicer arietinum L.). J Agron Crop Sci 195:47–54
Cernay C, Pelzer E, Makowski D (2016) A global experimental dataset for assessing grain legume production. Sci Data 3:160084
Chakraborty U, Pradhan D (2011) High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. J Plant Interact 6:43–52
Chakraborty U, Tongden C (2005) Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. Curr Sci 89:384–388
Chang MY, Chen SL, Lee CF, Chen YM (2001) Cold-acclimation and root temperature protection from chilling injury in chilling-sensitive mungbean (Vigna radiata L.) seedlings. Bot Bull Acad Sin 42:53–60
Chen S, Iyer A, Caplan DF, Klessig BF (1997) Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol 114:193–201
Chocobar-Ponce S, Prado C, González R, Rosa M, Prado FE (2014) Mitochondrial respiration: involvement of the alternative respiratory pathway and residual respiration in abiotic stress responses. In: Ahmed P, Wani MdR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment. Springer, New York, pp 321–364
Christen D, Schönmann S, Jermini M, Strasser RJ, Dèfago G (2007) Characterization and early detection of grapevine (Vitis vinifera) stress response to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ Exp Bot 60:504–514
Clarke HJ, Siddique KHM (2004) Response of chickpea genotypes to low temperature stress during reproductive development. Field Crops Res 90:323–334
Clarke HJ, Khan TN, Siddique KH (2004) Pollen selection for chilling tolerance at hybridisation leads to improved chickpea cultivars. Euphytica 139:65–74
Cloutier J, Prevost D, Nadeau P, Antoun H (1992) Heat and cold shock protein synthesis in arctic and temperate strains of rhizobia. Appl Environ Microbiol 58:2846–2853
Collins GG, Nie XL, Saltveit ME (1995) Heat-shock proteins and chilling sensitivity of mung bean hypocotyls. J Exp Bot 46:795–802
Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, Begara-Morales JC, Airaki M, del Río LA, Barroso JB (2008) Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol 49:1711–1722
Craufurd PQ, Summerfield RJ, Ell RH, Roberts EH (1997) Photoperiod, temperature and the growth and development of cowpea (Vigna unguiculata). In: Singh BB, Mohan Raj DR, Dashiell KE, Jackai LEN (eds) Advances in cowpea research. Co-publication Intl Inst Tropical Agric (IITA) and Japan Intl Res Center Agric Sci (JIRCAS), Sayce, Devon, UK, pp 75–86
Croser JS, Clarke HJ, Siddique KHM, Khan TN (2003) Low temperature stress: implications for chickpea (Cicer arietinum L.) improvement. Crit Rev Plant Sci 22:185–219
De Ronde JA, Cress WA, Kruger GHJ, Strasser RJ, Van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol 161:1211–1224
Devasirvatham V, Gaur PM, Mallikarjuna N, Raju TN, Trethowan RM, Tan DK (2013) Reproductive biology of chickpea response to heat stress in the field is associated with the performance in controlled environments. Field Crop Res 142:9–19
Dita MA, Rispail N, Prats E, Rubiales D, Singh KB (2006) Biotechnology approaches to overcome biotic and abiotic stress constraints in legumes. Euphytica 147:1–24
Djanaguiraman M, Prasad PVV, Al-Khatib K (2011) Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environ Exp Bot 71:215–223
Djanaguiraman M, Prasad PVV, Boyle DL, Schapaugh WT (2013) Soybean pollen anatomy, viability and pod set under high temperature stress. J Agron Crop Sci 199:171–177
Drouin P, Prevost D, Antoun H (2000) Physiological adaptation to low temperatures of strains of Rhizobium leguminosarum bv. viciae associated with Lathyrus spp. FEMS Microbiol Ecol 32:111–120
Duc G, Petitjean F (1995) Study of the inheritance of freezing tolerance in Vicia faba L. In: AEP (ed) Proceedings of 2nd European Conference on Grain Legumes, Copenhagen, AEP, Paris, pp 130–131
Duke JA (1981) Handbook of legumes of world economic importance. Plenum Press, New York, pp 52–57
El-Bassiony AM, Ghoname AA, El-Awadi ME, Fawzy ZF, Gruda N (2012) Ameliorative effects of brassinosteroids on growth and productivity of snap beans grown under high temperature. Gesunde Pflanzen 64:175–182
Ellis TH, Turner L, Hellens RP, Lee D, Harker CL, Enard C, Domoney C, Davies DR (1992) Linkage maps in pea. Genetics 130:649–663
Erskine W, Hussain A, Tahir M, Bahksh A, Ellis RH, Summerfield RJ, Roberts EH (1994) Field evaluation of a model of photothermal flowering responses in a world lentil collection. Theor Appl Genet 88:423–428
Esfandiari E, Shekari F, Shekari F, Esfandiari M (2007) The effect of salt stress on antioxidant enzymes activity and lipid peroxidation on the wheat seedling. Not Bot Hort Agrobot Cluj 35:48–56
Ferris R, Wheeler TR, Hadley P, Ellis RH (1998) Recovery of photosynthesis after environmental stress in soybean grown under elevated CO2. Crop Sci 38:948–955
Flandez-Galvez H, Ford R, Pang ECK, Taylor PWJ (2003) An intraspecific linkage map of the chickpea (Cicer arietinum L.) genome based on sequence tagged microsatellite site and resistance gene analog markers. Theorl Appl Genet 106:1447–1456
Fletcher HF, Ormrod DP, Maurer AR, Stanfield B (1966) Response of peas to environment; planting date and location. Can J Plant Sci 46:77–85
Funatsuki H, Matsuba S, Kawaguchi K, Murakami T, Sato Y (2004) Methods for evaluation of soybean chilling tolerance at the reproductive stage under artificial climatic conditions. Plant Breed 123:558–563
Gaur P, Saminen S, Krishnamurthy L et al (2015) High temperature tolerance in grain legumes. Legume Perspect 7:23–24
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
Georgieva K, Lichtenthaler HK (2006) Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica 44:569–578
Goltsev V, Yordanov I, Gurmanova M, Peshev I, Strasser RJ (2010) Temperature-induced transitions in photosynthetic apparatus of bean plants probed by JIP-test. Agrar Nauki 2:7–14
Gomes DF, Batista JSS, Schiavon AL, Andrade DS, Hungria M (2012) Proteomic profiling of Rhizobium tropici PRF 81: identification of conserved and specific responses to heat stress. BMC Microbiol 12:84
Gonzalez-Meler MA, Ribascarbo M, Giles L, Siedow JN (1999) The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol 120:765–772
Gopalakrishnan S, Dudeja SS (1999) Temperature tolerance and effectiveness of Rhizobium strains infecting pigeon pea (Cajanus cajan L.) host. Indian J Plant Physiol 4:6–9
Graham PH, Vance CP (2003) Legumes: importance and constraints to greater use. Plant Physiol 131:872–877
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Gross Y, Kigel J (1994) Differential sensitivity to high temperature stress in the reproductive development of common bean (Phaseolus vulgaris L.). Field Crops Res 36:201–212
Guye MG, Vigh L, Wilson JM (1986) Polyamine titre in relation to chilling-sensitivity in Phaseolus sp. J Exp Bot 37:1036–1043
Haldimann P, Feller U (2005) Growth at moderately elevated temperature alters the physiological response of the photosynthetic apparatus to heat stress in pea (Pisum sativum L.) leaves. Plant Cell Environ 28:302–317
Hamada AM (2001) Alteration in growth and some metabolic processes of broad bean plants during extreme temperatures exposure. Acta Physiol Plant 23:193–200
Hamdi A, Küsmenoĝlu I, Erskine W (1996) Sources of winter hardiness in wild lentil. Genet Resour Crop Evol 43:63–67
Harlan JR, de Wet JM (1971) Towards a rational classification of cultivated plants. Taxon 20:509–517
Hasanuzzaman M, Nahar K, Fujita M (2013) Extreme temperature responses, oxidative stress and antioxidant defense in plants. In: Vahdati K, Leslie C (eds) Abiotic stress–plant responses and applications in agriculture. InTech, Vienna, pp 169–205
Hatfield JL, Boote KJ, Kimball BA et al (2011) Climate impacts on agriculture: implications for crop production. Agron J 103:351–370
Hawtin GC, Muehlbauer FJ, Slinkard AE, Singh KB (1988) Current status of cool season food legume crop improvement: an assessment of critical needs. In: Summerfield RJ (ed) World crops: cool season food legumes. Kluwer Academic Publishers, Dordrecht, pp 67–80
Hedhly A, Hormaza JI, Herrero M (2008) Global warming and plant sexual reproduction. Trends Plant Sci 14:30–36
Heidarvand L, Amiri RM, Naghavi MR, Farayedi Y, Sadeghzadeh B, Alizadeh KH (2011) Physiological and morphological characteristics of chickpea accessions under low temperature stress. Russ J Plant Physiol 58:157–163
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Horváth I, Glatz A, Nakamoto H, Mishkind ML, Munnik T, Saidi Y, Goloubinoff P, Harwood JL, Vigh L (2012) Heat shock response in photosynthetic organisms membrane and lipid connections. Prog Lipid Res 51:208–220
Horwich AL, Fenton WA (2009) Chaperonin-mediated protein folding: using a central cavity to kinetically assist polypeptide chain folding. Q Rev Biophys 42:83–116
Huang B, Chu CH, Chen SL, Juan HF, Chen YM (2006) A proteomics study of the mung bean epicotyl regulated by brassinosteroids under conditions of chilling stress. Cell Mol Biol Lett 11:264–278
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 -fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res 65:151–164
Hungria M, Franco AA, Sprent JJ (1993) New sources of high temperature tolerant rhizobia for Phaseolus vulgaris L. Plant Soil 149:103–109
Ibrahim HM (1994) Tolerance and adaptation of chickpea to heat stress. Dissertation, Alexandria University
Ibrahim HM (2011) Heat stress in food legumes: evaluation of membrane thermostability methodology and use of infra-red thermometry. Euphytica 180:99–105
Ishikawa HA (1996) Ultrastructural features of chilling injury: injured cells and the early events during chilling of suspension cultured mungbean cells. Am J Bot 83:825–835
Ismail AM, Hall AE (1999) Reproductive stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop Sci 39:1762–1768
Jakobsen HB, Martens H (1994) Influence of temperature and aging of ovules and pollen on reproductive success in Trifolium repens L. Ann Bot 74:493–501
Jewell MC, Campbell BC, Ian D (2010) Transgenic plants for abiotic stress resistance. In: Chittaranjan K, Charles HM, Albert GA, Timothy CH (eds) Transgenic crop plants. Springer, Berlin, pp 67–132
Jiang Y, Huang B (2001) Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J Exp Bot 52:341–349
Kahraman A, Kusmenoglu I, Aydin N, Aydogan A, Erskine W, Muehlbauer FJ (2004) QTL mapping of winter hardiness genes in lentil. Crop Sci 44:13–22
Kakani VG, Reddy KR, Koti S, Wallace TP, Prasad PVV, Reddy VR, Zhao D (2005) Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann Bot 96:59–67
Karim MA, Fracheboud Y, Stamp P (1997) Heat tolerance of maize with reference of some physiological characteristics. Ann Bangladesh Agric 7:27–33
Kaur G, Kumar S, Nayyar H, Upadhyaya HD (2008) Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): effects on quantitative and qualitative components of seeds. J Agron Crop Sci 194:457–464
Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, Nayyar H (2011) Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol Mol Biol Plants 17:203–213
Kaushal N, Awasthi R, Gupta K, Gaur P, Siddique KH, Nayyar H (2013) Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct Plant Biol 40:1334–1349
Kigel J, Konsens I, Ofir M (1991) Branching, flowering and pod-set patterns in snap-bean (Phaseolus vulgaris L.) as affected by temperature. Can J Plant Sci 71:1233–1242
Kishinevsky BD, Sen D, Weaver RW (1992) Effect of high root temperature on Bradyrhizobium-peanut symbiosis. Plant Soil 143:275–282
Konsens I, Ofir M, Kigel J (1991) The effect of temperature on the production and flowers and pods in snap bean (Phaseolus vulgaris L.). Ann Bot 67:391–399
Koti S, Reddy KR, Kakani VG, Zhao D, Reddy VR (2004) Soybean (Glycine max) pollen germination characteristics, flower and pollen morphology in response to enhanced ultraviolet-B radiation. Ann Bot 94:855–864
Kratsch HA, Wise RR (2000) The ultrastructure of chilling stress. Plant Cell Environ 23:337–350
Krishnamurthy L, Gaur PM, Basu PS et al (2011) Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet Res 9:59–69
Kumar A, Omae H, Kashiwaba K, Shono M (2008a) Dry matter partitioning in heat tolerant and sensitive snap bean cultivars under shifting air temperatures. Indian J Plant Physiol 13:191–194
Kumar S, Kaur G, Nayyar H (2008b) Exogenous application of abscisic acid improves cold tolerance in chickpea (Cicer arietinum L.). J Agron Crop Sci 194:449–456
Kumar S, Nayyar H, Bhanwara RK, Upadhyaya HD (2010) Chilling stress effects on reproductive biology of chickpea. J SAT Agric Res 8:1–14
Kumar S, Kaur R, Kaur N, Bhandhari K, Kaushal N, Gupta K, Bains TS, Nayyar H (2011a) Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol Plant 33:2091–2101
Kumar S, Malik J, Thakur P, Kaistha S, Sharma KD, Upadhyaya HD, Berger JD, Nayyar H (2011b) Growth and metabolic responses of contrasting chickpea (Cicer arietinum L.) genotypes to chilling stress at reproductive phase. Acta Physiol Plant 33:779–787
Kumar S, Kaushal N, Nayyar H, Gaur P (2012) Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol Plant 34:1651–1658
Kumar S, Thakur P, Kaushal N, Malik JA, Gaur P, Nayyar H (2013) Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch Agron Soil Sci 59:823–843
Kurosaki H, Yumoto S (2003) Effects of low temperature and shading during flowering on the yield components in soybeans. Plant Prod Sci 6:17–23
Laranjo M, Oliveira S (2011) Tolerance of Mesorhizobium type strains to different environmental stresses. Antonie van Leeuwenhoek 99:651–662
Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Zhu JK (2002) LOS2, a genetic locus required for cold responsive transcription encodes a bi-functional enolase. EMBO J 21:2692–2702
Lei T, Feng H, Sun X, Dai QL, Zhang F, Liang HG, Lin HH (2010) The alternative pathway in cucumber seedlings under low temperature stress was enhanced by salicylic acid. Plant Growth Regul 60:35–42
Leshem YY (2001) Nitric Oxide in Plants. Kluwer Academic Publishers, London
Leshem YY, Haramaty E, Iluz D, Malik Z, Sofer Y, Roitman L, Leshem Y (1997) Effect of stress nitric oxide (NO): interaction between chlorophyll fluorescence, galactolipid fluidity and lipoxygenase activity. Plant Physiol Biochem 35:573–579
Li M, Li Z, Li S, Guo S, Meng Q, Li G, Yang X (2014) Genetic engineering of glycine betaine biosynthesis reduces heat-enhanced photoinhibition by enhancing antioxidative defense and alleviating lipid peroxidation in tomato. Plant Mol Biol Rep 32:42–51
Link W, Balko C, Stoddard FL (2010) Winter hardiness in faba bean: physiology and breeding. Field Crop Res 115:287–296
Lira MD, Lima AST, Arruda JRF, Smith DL (2005) Effect of root temperature on nodule development of bean, lentil and pea. Soil Biol Biochem 37:235–239
Lynch DH, Smith DL (1993) Soybean (Glycine max) nodulation and N2-fixation as affected by exposure to a low root-zone temperature. Physiol Plant 88:212–220
Ma SF, Lin CY, Chen YM (1990) Comparative studies of chilling stress on alteration of chloroplasts ultrastructure and protein synthesis in leaves of chilling-sensitive (mungbean) and insensitive (pea) seedlings. Bot Bull Acad Sin 31:263–272
Mabood F, Smith D (2007) The role of salicylates in Rhizobium-legume symbiosis and abiotic stresses in higher plants. In: Hayat S, Ahmad A (eds) Salicylic acid. A plant hormone. Springer Publishers, Dordrecht, pp 151–162
Mahoney J (1991) Field pea. In: Jessop RS, Wright RL (eds) New crops: agronomy and potential of alternative crop species. Inkata Press, Melbourne, pp 53–62
Malhotra RS (1998) Breeding chickpea for cold tolerance. In: 3rd European Conference on grain legumes. Opportunities for high quality, healthy and added-value crops to meet European demands, Valladolid, European Association for Grain Legume Research, Paris, France, p 152
Mansoor S, Naqvi FN (2011) Heat stress and acquisition of thermotolerance in mung bean (Vigna radiata L.). Int J Biol Biotechnol 8:77–84
Mansoor S, Naqvi FN (2013) Effect of heat stress on lipid peroxidation and antioxidant enzymes in mungbean (Vigna radiata L) seedlings. Afr J Biotechnol 12:3196–3203
Maqbool A, Shafiq S, Lake L (2010) Radiant frost tolerance in pulse crops—a review. Euphytica 172:1–12
McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inze D, D'Halluin K, Botterman J (1993) Superoxide Dismutase Enhances Tolerance of Freezing Stress in Transgenic Alfalfa (Medicago sativa L.). Plant Physiol 103:1155–1163
McKersie BD, Murnaghan J, Jones KS, Bowley SR (2000) Iron-Superoxide Dismutase Expression in Transgenic Alfalfa Increases Winter Survival without a Detectable Increase in Photosynthetic Oxidative Stress Tolerance. Plant Physiol 122:1427–1438
Mingpeng H, Yongge G, Chengzhang W, Fangrui S, Yanhua W, Xiaoxia Z (2010) Related studies on the effects of high temperature stress on alfalfa and its heat resistance mechanism. Genom Appl Biol 29:563–569
Moawad H, Beck D (1991) Some characteristics of Rhizobium leguminosarum isolates from uninoculated field-grown lentil. Soil Biol Biochem 23:917–925
Mohammed AR, Tarpley L (2009) Impact of high nighttime temperature on respiration, membrane stability, antioxidant capacity, and yield of rice plants. Crop Sci 49:313–322
Mohapatra SM, Poole RJ, Dhindsa RS (1988) Abscisic acid-regulated gene expression in relation to freezing tolerance in alfalfa. Plant Physiol 87:468–473
Muehlbauer FJ, Kaiser WJ, Kutlu Z, Sperling CR (1990) Collection of Pisum germplasm in Turkey in 1985 and 1989. Pisum Newsl 22:97–98
Muehlbauer FJ, Kaiser WJ, Simon CJ (1994) Potential for wild species in cool season food legume breeding. Euphytica 73:109–114
Munro KD, Hodges DM, DeLong JM, Forney CF, Kristie DN (2004) Low temperature effects on ubiquinone content, respiration rates and lipid peroxidation levels of etiolated seedlings of two differentially chilling-sensitive species. Physiol Plant 12:488–497
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015) Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot 112:44–54
Nayyar H (2005) Putrescine increases floral retention, pod set and seed yield in cold stressed chickpea. J Agron Crop Sci 191:340–345
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365
Nayyar H, Bains TS, Kumar S (2005a) Chilling stressed chickpea seedlings: effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ Exp Bot 54:275–285
Nayyar H, Bains TS, Kumar S (2005b) Low temperature induced floral abortion in chickpea: relationship to abscisic acid and cryoprotectants in reproductive organs. Environ Exp Bot 53:39–47
Nayyar H, Bains TS, Kumar S, Kaur G (2005c) Chilling effects during seed filling on accumulation of seed reserves and yield of chickpea. J Sci Food Agric 85:1925–1930
Nayyar H, Chander K, Kumar S, Bains T (2005d) Glycine betaine mitigates cold stress damage in Chickpea. Agron Sustain Dev 25:381–388
Nayyar H, Kaur G, Kumar S, Upadhyaya HD (2007) Low temperature effects during seed filling on chickpea genotypes (Cicer arietinum L.): probing mechanisms affecting seed reserves and yield. J Agron Crop Sci 193:336–344
Nehra K, Yadav AS, Sehrawat A, Vashishat RK (2007) Characterization of heat resistant mutant strains of Rhizobium sp. [Cajanus] for growth, survival and symbiotic properties. Indian J Microbiol 47:329–335
Niste M, Vidican R, Pop R, Rotar I (2013) Stress factors affecting symbiosis activity and nitrogen fixation by Rhizobium cultured in vitro. ProEnvironment 6:42–45
O’Connell KP, Thomashow MF (2000) Transcriptional organization and regulation of a polycistronic cold shock operon in Sinohizobium meliloti RM1021 encoding homologs of the Escherichia coli major cold shock gene cspA and ribosomal protein gene rpsU. Appl Environ Microbiol 66:392–400
Ohnishi S, Miyoshi T, Shirai S (2010) Low temperature stress at different flower developmental stages affects pollen development, pollination, and pod set in soybean. Environ Exp Bot 69:56–62
Oliver SN, Van Dongen JT, Alfred SC et al (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ 28:1534–1551
Olszewski A (1996) Optimization of frost damage determination using the chlorophyll fluorescence method and its use for a frost resistance screening at Vicia faba L. Shaker Verlag, Aachen, Germany
Omae H, Kumar A, Kashiwaba K, Shono M (2005) Influence of level and duration of high temperature treatments on plant water status in snap bean (Phaseolus vulgaris). Jpn J Trop Agric 49:238–242
Omae H, Kumar A, Kashiwaba K, Shono M (2007) Influence of temperature shift after flowering on dry matter partitioning in two cultivars of snap bean (Phaseolus vulgaris) that differ in heat tolerance. Plant Prod Sci 10:14–19
O'Toole N, Stoddard FL, O'Brien L (2001) Screening of chickpeas for adaptation to autumn sowing. J Agron Crop Sci 186:193–207
Pardha Saradhi P, Sharmila P (2003) Improvement of crop plants for abiotic stress tolerance through introduction of glycinebetaine pathway. In: Singh RP, Jaiwal PK (eds) Plant genetic engineering. SCI Tech Publishing, Houston, USA, pp 147–177
Perl-Treves R, Perl A (2002) Oxidative stress: an introduction. In: Inzè D, Van Montagu M (eds) Oxidative stress in plants. Taylor & Francis, London, New York, pp 1–32
Petkova V, Denev ID, Cholakov D, Porjazov I (2007) Field screening for heat tolerant common bean cultivars (Phaseolus vulgaris L.) by measuring of chlorophyll fluorescence induction parameters. Sci Hortic 111:101–106
Piramila BHM, Prabha AL, Nandagopalan V, Stanley AL (2012) Effect of heat treatment on germination, seedling growth and some biochemical parameters of dry seeds of black gram. Int J Pharm Phytopharmacol Res 1:194–202
Poehlman JM (1991) A scanning electron microscopic study on the influence of temperature on the expression of cytoplasmic male sterility in Brassica napus. Can J Bot 65:807–814
Popelka JC, Terryn N, Higgins TJV (2004) Gene technology for grain legumes: can it contribute to the food challenge in developing countries? Plant Sci 167:195–206
Porch TG, Jahn M (2001) Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Environ 24:723–731
Porter JR, Semenov MA (2005) Crop responses to climatic variation. Philos T R Soc B 360:2021–2035
Posmyk MM, Janas KM (2007) Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiol Plant 29:509–517
Posmyk MM, Bailly C, Szafran´ska K, Janasa KM, Corbineau F (2005) Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings. J Plant Physiol 162:403–412
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK (2007) Stress-induced morphogenic responses: growing out of trouble. Trends Plant Sci 12:98–105
Prasad PVV, Craufurd PQ, Summerfield RJ, Wheeler TR (2000) Effects of short episodes of heat stress on flower production and fruit-set of groundnut (Arachis hypogaea L.). J Exp Bot 51:777–784
Prévost D, Drouin P, Antoun H (1999) The potential use of cold-adapted rhizobia to improve symbiotic nitrogen fixation in legumes cultivated in temperate regions. In: Margesin R, Schinner F (eds) Biotechnological applications of cold-adapted organisms. Springer-Verlag, Berlin, pp 161–176
Raison JK, Orr GR (1986) Phase transitions in thylakoid polar lipids of chilling-sensitive plants: a comparison of detection methods. Plant Physiol 80:638–645
Rajesh PN, Coyne C, Meksem K et al (2004) Construction of a Hin dIII Bacterial Artificial Chromosome library and its use in identification of clones associated with disease resistance in chickpea. Theor Appl Genet 108:663–669
Redden R, Palta-Paz J, Atkins C, Gready JE, Dwyer SA, Evans JR (2013) Legume productivity and photosynthetic responses anticipated with climate change: insights from lupins. In: Gready JE, Dwyer SA, Evans JR (eds) Applying photosynthesis research to improvement of food crops. ACIAR Publications, Fern Hill Park BRUCE ACT, Australia, pp 61–144
Reddy S, Bhatnagar-Mathur P, Vadez V, Sharma KK (2012) Grain Legumes (Soybean, Chickpea, and Peanut): Omics Approaches to Enhance Abiotic Stress Tolerance. In: Tuteja N, Gill SS, Tiburcio AF, Tuteja R (eds) Improving Crop Resistance to Abiotic Stress, 1st edn. Wiley-VCH Verlag GmbH & Co. KGaA, Singapore.
Repo T, Mononen K, Alvila L, Pakkanen TT, Hanninen H (2008) Cold acclimation of pedunculate oak (Quercus robur L.) at its northern most distribution range. Environ Exp Bot 63:59–70
Ribbe MW, Burgess BK (2001) The chaperone GroEL is required for the final assembly of the molybdenum-iron protein of nitrogenase. Proc Natl Acad Sci USA 98:5521–5525
Rice WA, Olsen PE (1988) Root-temperature effects on competition for nodule occupancy between two Rhizobium meliloti strains. Biol Fert Soils 6:137–140
Robertson AJ, Ishikawa M, Gusta LV, MacKenzie SL (1994) Abscisic acid induced heat tolerance in Bromus inermis Leyss cell suspension cultures. Plant Physiol 105:181–190
Roy CD, Tarafdar S, Das M, Kundagrami S (2012) Screening lentil (Lens culinaris Medik.) germplasms for heat tolerance. Trends Biosci 5:143–146
Rustogi N, Badhwar S, Sharma PK, Dogra RC (1996) Temperature tolerance and efficiency of Rhizobium sp. (Acacia) in symbiosis with Acacia nilotica. In: Dogra RC, Behl RK, Khurana AL (eds) Resource management in agriculture. CCS Haryana Agric University, Hisar and Max Muller Bhavan, New Delhi, pp 107–112
Sainz M, Dıaz P, Monza J, Borsan O (2010) Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought-heat stressed Lotus japonicus. Physiol Plant 140:46–56
Saleh AA (2007) Amelioration of chilling injuries in mung bean (Vigna radiata L.) seedlings by paclobutrazol, abscisic acid and hydrogen peroxide. Am J Plant Physiol 2:318–332
Salem MA, Kakan VG, Koti S, Reddy KR (2007) Pollen-based screening of soybean genotypes for high temperatures. Crop Sci 47:219–231
Sarker A, Aydin N, Aydogan A, Sabaghpour SH, Ketata H, Kusmenoglu I, Erskine W (2002) Winter lentils promise improved nutrition and income in west Asian highlands. ICARDA Caravan 16:14–16
Sandhu JS, Gupta SK, Singh S, Dua RP (2007) Genetic variability for cold tolerance in pigeon pea. J SAT Agric Res 5:1–3
Sardesai N, Babu CR (2001) Cold stress induced high molecular weight membrane polypeptides are responsible for cold tolerance in Rhizobium DDSS69. Microbiol Res 156:279–284
Sato S, Peet MM, Thomas JF (2000) Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant Cell Environ 23:719–726
Sato S, Nakamura Y, Kaneko T et al (2008) Genome Structure of the Legume, Lotus japonicus. DNA Res 15:227–239
Schmutz J, Cannon SB, Schlueter J et al (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Schuster WS, Monson RK (1990) An examination of the advantages of C3–C4 intermediate photosynthesis in warm environments. Plant, Cell Environ 13:903–912
Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul 30:157–161
Shafiq S, Diane E, Mather DE, Ahmad M, Paull JG (2012) Variation in tolerance to radiant frost at reproductive stages in field pea germplasm. Euphytica 186:831–845
Sharma K, Nayyar H (2014) Cold stress alters transcription in meiotic anthers of cold tolerant chickpea (Cicer arietinum L.). BMC Res Notes 7:717
Shereena J, Salim N (2006) Chilling tolerance in Pisum sativum L. seeds: an ecological adaptation. Asian J Plant Sci 5:1047–1050
Shimoda Y, Nagata M, Suzuki A, Abe M, Sato S, Kato T, Tabata S, Higashi S, Uchiumi T (2005) Symbiotic Rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol 46:99–107
Simon CJ, Muehlbauer FJ (1991) Expansion of the chickpea and lentil linkage map using morphological, isozyme, RFLP, and RAPD markers. In: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America (eds) Agronomy abstracts, American Society of Agronomy, Madison, Wisconsin, USA, pp 116
Simon CJ, Tahir M, Muehlbauer FJ (1993) Linkage map of lentil (Lens culinaris) 2n = 14. In: O'Brien SJ (ed) Genetic maps, Locus maps of complex genomes, 6th edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, pp 96–100
Singh NH, Dhaliwal GS (1972) Effect of soil temperature on seedling emergence in different crops. Plant Soil 37:441–447
Singh KB, Malhotra RS, Halila H, Knights EJ, Verma MM (1994) Current status for future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 73:137–149
Singh KB, Malhotra RS, Saxena MC (1995) Additional sources of tolerance to cold in cultivated and wild Cicer species. Crop Sci 35:1491–1497
Singh KB, Omar M, Saxena MC, Johansen C (1996) Registration of FLIP 87-59C, a drought-tolerant chickpea germplasm line. Crop Sci 36:472
Singh NK, Gupta DK, Jayaswal PK et al (2012) The first draft of the pigeonpea genome sequence. J Plant Biochem Biotechnol 21:98–112
Srikanthbabu V, Krishnaprasad BT, Gopalakrishna R, Savitha M, Udayakumar M (2002) Identification of pea genotypes with enhanced thermotolerance using temperature induction response technique (TIR). J Plant Physiol 159:535–545
Srinivasan A, Takeda H, Senboku T (1996) Heat tolerance in food legumes as evaluated by cell membrane thermostability and chlorophyll fluorescence techniques. Euphytica 88:35–45
Srinivasan A, Saxena NP, Johansen C (1999) Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L): genetic variation in gamete development and function. Field Crops Res 60:209–222
Srivastava MK, Dwivedi UN (1998) Salicylic acid modulates glutathione metabolism in pea seedlings. J Plant Physiol 153:404–409
Srivastava V, Soni A, Sonam K (2016) Analysis on effect of cold stress in bean seeds (Phaseolus vulgaris L.). Am J Biosci 3:145–166
Stoddard FL, Balko C, Erskine W, Khan HR, Link W, Sarker A (2006) Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 147:167–186
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P (ed) Photosynthesis: from light to biosphere. Kluwer Academic, The Netherlands, pp 977–980
Suárez R, Calderón C, Iturriaga G (2009) Enhanced Tolerance to Multiple Abiotic Stresses in Transgenic Alfalfa Accumulating Trehalose. Crop Sci 49:1791
Suzuki K, Tsukaguchi T, Takeda H, Egawa Y (2001) Decrease of pollen stainability of green bean at high temperatures and relationship to heat tolerance. J Am Soc Hortic Sci 126:571–574
Suzuki K, Shono M, Egawa Y (2003) Occurrence of abnormal pods and abscission of flowers at high temperatures in snap bean. Res Highlights JIRCAS 2002:46–47
Szalai G, Pál M, Janda T (2011) Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. Acta Biol Szeged 55:155–157
Talwar HS, Takeda H, Yashima S, Senboku T (1999) Growth and photosynthetic responses of groundnut genotypes to high temperature. Crop Sci 39:460–466
Tambussi EA, Bartoli CG, Guiamet JJ, Beltrano J, Araus JL (2004) Oxidative stress and photodamage at low temperatures in soybean (Glycine max L. Merr.) leaves. Plant Sci 167:19–26
Tang RS, Zheng JC, Jin ZQ, Zhang D, Huang H, Chen LG (2008) Possible correlation between high temperature induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54:37–43
Teplova IR, Farkhutdinov RG, Mitrichenko AN, Ivanov II, Veselov SY, Valcke RL, Kudoyarova GR (2000) Response of tobacco plants transformed with the ipt gene to elevated temperature. Russ J Plant Physiol 47:367–369
Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H (2010) Cold stress effects on reproductive development in grain crops, an overview. Environ Exp Bot 67:429–443
Thiaw S, Hall AE (2004) Comparison of selection for either leaf electrolyte leakage or pod set in enhancing heat tolerance and grain yield of cowpea. Field Crops Res 86:239–253
Todorova D, Katerova Z, Shopova E, Jodinskiene M, Jurkoniene S, Sergiev I (2016) Responses of pea plants to heat stress and spermine treatment. Zemdirbyste 103:99–106
Toker C, Cagirgan MI (1998) Assessment of response to drought stress of chickpea (Cicer arietinum L.) lines under rainfed conditions. Turk J Agric For 22:615–621
Tsonev T, Velikova V, Georgieva K, Hyde PF, Jones HG (2003) Low temperature enhances photosynthetic down-regulation in French bean (Phaseolus vulgaris L.) plants. Ann Bot 91:343–352
Tsukaguchi T, Egawa Y (2006) Higher stability in leaf water status in heat-tolerant cultivars of snap bean (Phaseolus vulgaris L.). Bull Fac Agric Niigata Univ 58:79–84
Turan O, Ekmekçi Y (2011) Activities of photosystem II and antioxidant enzymes in chickpea (Cicer arietinum L.) cultivars exposed to chilling temperatures. Acta Physiol Plant 33:67–78
Upreti KK, Murti GSR (2004) Effects of brassinosteroids on growth, nodulation, phytohormone content and nitrogenase activity in French bean under water stress. Biol Plant 48:407–411
Upreti KK, Murti GSR, Bhatt RM (2000) Response of pea cultivars to water stress: changes in morpho-physiological characters, endogenous hormones and yield. Vegetable Sci 27:57–61
Urbatzka P, Graß R, Schüler C (2005) Use of plant genetic resources in organic agriculture—the example of winter pea. In: Heß J, Rahmann G (eds) Scientific Conference on Organic Agriculture “Ende der Nische”, 1–4 March 2005, Kassel University, Kassel, Germany, pp 59–60
Van der Maesen LJG, Pundir RPS (1984) Availability and use of wild Cicer germplasm. Plant Genet Resour Newsl 57:19–24
Van Heerden PDR, Krűger GHJ (2000) Photosynthetic limitation in soybean during cold stress. S Afr J Sci 96:201–206
Van Heerden PDR, Krüger GHJ, Loveland JE, Parry MAJ, Foyer CH (2003) Dark chilling imposes metabolic restrictions on photosynthesis in soybean. Plant Cell Environ 26:323–337
Van Heerden PDR, Strasser RJ, Kruger GHJ (2004) Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol Plant 121:239–249
Van Heerden PDR, Kiddle G, Pellny TK et al (2008) Regulation of respiration and the oxygen diffusion barrier in soybean protect symbiotic nitrogen fixation from chilling-induced inhibition and shoots from premature senescence. Plant Physiol 148:316–327
Vance CP, Graham PH, Allan DL (2000) Biological nitrogen fixation. Phosphorus: a critical future need. In: Pedrosa FO, Hungria M, Yates MG, Newton WE (eds) Nitrogen fixation: from molecules to crop productivity. Kluwer Academic Publishers, Dordrecht, pp 506–514
Vanlerberghe GC (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci 14:6805–6847
Vardhini BV, Rao SSR (1998) Effect of brassinosteroids on growth, metabolite content and yield of Arachis hypogaea. Phytochemistry 48:927–930
Varshney RK, Chen W, Li Y, Bharti AK et al (2011) Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nature Biotechnol 30:83–89
Varshney RK, Song C, Saxena RK (2013) Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nature Biotechnol 31:240–246
Verma MM, Sandhu JS, Rraar HS, Brar JS (1990) Crossability studies in different species of Cicer (L.). Crop Improv 17:179–181
Vogan AA, Higgs PG (2011) The advantages and disadvantages of horizontal gene transfer and the emergence of the first species. Biol Direct 6:1
Wahid A, Gelani S, Ashraf M, Foolad M (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang J, Gan YT, Clarke F, McDonald CL (2006) Response of chickpea yield to high temperature stress during reproductive development. Crop Sci 46:2171–2178
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Differential antioxidation activities in two alfalfa cultivars under chilling stress. Plant Biotechnol Rep 3:301–307
Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W (2010) Over-accumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 48:30–41
Weeden NF, Wolko B (1990) Linkage map for the garden pea (Pisum sativum) based on moleculars markers. In: O'Brien SJ (ed) Genetic maps, 5th edn. Cold Spring Harbor Laboratory Cold Spring Harbor, New York, pp 6106–6112
Weeden NF, Muehlbauer FJ, Ladizinsky G (1992) Extensive conservation of linkage relationships between pea and lentil genetic maps. J Hered 83:123–129
Wilson JM (1987) Chilling injury in plants. In: Grout BWW, Morns GJ (eds) The effects of low temperatures on biological systems. Edward Arnold Publishers, London, pp 271–292
Wise R, Olson A, Schrader S, Sharkey TD (2004) Electron transport is the functional limitation of photosynthesis in field-grown pima cotton plants at high temperature. Plant Cell Environ 27:717–724
Xu S, Li J, Zhang X, Wei H, Cui L (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Yadav AS (2008) Resistance to sodium azide and its effect on symbiotic effectivity of rhizobial strains: a review. Natl J Pl Improv 10:75–79
Yadav S, Nehra K (2013) Selection/Isolation of high temperature tolerant strains of Rhizobium for management of high temperature stress on Rhizobium–legume symbiosis. Int J Microb Resour Technol 2:47–57
Yadegari LZ, Heidari R, Carapetian J (2008) Chilling pre-treatment causes some changes in respiration, membrane permeability and some other factors in soybean seedlings. Res J Biol Sci 3:1054–1059
Yamada T, Kuroda K, Jitsuyama Y, Takezawa D, Arakawa K, Fujikawa S (2002) Roles of the plasma membrane and the cell wall in the responses of plant cells to freezing. Planta 215:770–778
Yan SP, Zhang QY, Tang ZC, Su WA, Sun WN (2006) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteom 5:484–496
Yang XH, Liang Z, Lu CM (2005) Genetic engineering of the biosynthesis of glycine betaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138:2299–2309
Yang JD, Yun JY, Zhang TH, Zhao HL (2006) Presoaking with nitric oxide donor SNP alleviates heat shock damages in mung bean leaf discs. Bot Stud 47:129–136
Young ND, Debellé F, Oldroyd GED et al (2011) The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480:520–524
Zhang JH, Huang WD, Liu YP, Pan QH (2005) Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under cross-temperature stresses. J Integr Plant Biol 47:959–970
Zhang LL, Zhao MG, Tian QY, Zhang WH (2011) Comparative studies on tolerance of Medicago truncatula and Medicago falcata to freezing. Planta 234:445–457
Zhao Y, Aspinall D, Paleg LG (1992) Protection of membrane integrity in Medicago sativa L. by glycine betaine against the effects of freezing. J Plant Physiol 140:541–543
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Zhuo C, Wang T, Lu S, Zhao Y, Li X, Guo Z (2013) A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by -inositol and confers multiple tolerances to abiotic stresses. Physiol Plant 149:67–78
Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61:1959–1968
Acknowledgements
The authors thank DST (Department of Science and Technology), UGC (University Grants Commission), DBT (Department of Biotechnology), New Delhi, ICARDA, Morocco, ICRISAT, India, and World Vegetable Center, Hyderabad, India, for the financial support for working on food legumes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. K. Kononowicz.
Rights and permissions
About this article
Cite this article
Bhandari, K., Sharma, K.D., Hanumantha Rao, B. et al. Temperature sensitivity of food legumes: a physiological insight. Acta Physiol Plant 39, 68 (2017). https://doi.org/10.1007/s11738-017-2361-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2361-5