Abstract
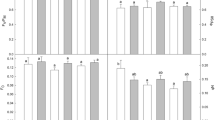
The thermo-sensitivity of two new pea (Pisum sativum L.) cultivars—Afila (mutant in the gene transforming leaves into mustaches) and Ranen (mutant for early ripening)—as compared to the control cultivar Pleven-4 to either low (4 °C, T4) or high temperature (38 °C, T38) was investigated by means of chlorophyll (Chl) fluorescence kinetics. The low temperature treatment decreased the photosynthetic activity, measured via a decline of the Chl fluorescence decrease ratios RFd690 and RFd735, and this was mainly due to a decline of the Chl fluorescence decrease parameter Fd and maximum Chl fluorescence Fm. In the new cv. Ranen the RFd ratios at first decreased and increased again after 24-h exposure to 4 °C, indicating its good acclimation ability to low temperature. The cold-induced changes in the photosynthetic performance of all cultivars were reversed after transferring plants back to 23 °C for 48 h. In the Chl and carotenoid (Car) contents no or little changes occurred during the T4 treatment, except for a slight but clear increase of the ratio Chl a/b and a decrease in the ratio Chl/Car. In contrast to this, the T38 treatment for 72 h decreased the RFd ratios more strongly than the T4 exposure did. In fact, an irreversible injury of the photosynthetic apparatus was caused in the control pea cv. Pleven-4 by a 48-h T38 exposure and for the new cv. Afila after a 72-h T38 exposure. In contrast, the cv. Ranen was less and little sensitive to the T38 exposure. In the heat-sensitive cvs. Pleven-4 and Afila, the decrease in RFd values at T38 was associated with a strong decline of the Chl a+b and total Car contents. The Chl a+b decline could also be followed via an increase of the Chl fluorescence ratio F690/F735. Parallel to this, a strong decline of Chl a/b from ca. 3.0 (range 2.85–3.15) to ca. 1.9 (range 1.85–1.95) occurred indicating a preferential decline of the Chl a-pigment proteins but not of the Chl a/b-pigment protein LHC2. In the relatively heat-tolerant cv. Ranen, however, the ratio Chl a/b declined only partially. After the T4 treatment the stress adaptation index Ap was higher in cv. Ranen than in controls and reached in heat-treated Ranen plants almost the starting value indicating a cold and heat stress hardening of the treated plants. The Chl fluorescence parameters and pigment contents were influenced by T38 and T4 treatments in various ways indicating that the mechanisms of low and high temperature injury of the photosynthetic apparatus are different. The new cv. Ranen exhibited a cross tolerance showing a fairly good acclimation ability to both T4 and T38, hence it is a very suitable plant for outdoor growth and for clarification of the acclimation mechanisms to unfavourable temperatures.
Similar content being viewed by others
Abbreviations
- Ap:
-
stress adaptation index
- Car:
-
carotenoid
- Chl:
-
chlorophyll
- cv.:
-
cultivar
- Fm :
-
maximal Chl fluorescence
- Fs :
-
steady state Chl fluorescence
- F690 :
-
red Chl fluorescence band near 690 nm
- F735 :
-
far-red Chl fluorescence band near 735 nm
- F680/F735 :
-
ratio of red to far-red Chl fluorescence
- PS:
-
photosystem
- RFd :
-
Chl fluorescence decrease ratio, measured at red (RFd690) and far-red Chl fluorescence maximum (RFd735), respectively
- T4 and T38 :
-
low and high temperature treatments at 4 and 38 °C, respectively
References
Babani, F., Lichtenthaler, H.K.: Light-induced and age-dependent development of chloroplasts in etiolated barley leaves as visualized by determination of photosynthetic pigments, CO2 assimilation rates and different kinds of chlorophyll fluorescence ratios.-J. Plant Physiol. 148: 555–566, 1996.
Berry, J., Björkman, O.: Photosynthetic response and adaptation to temperature in higher plants.-Annu. Rev. Plant Physiol. 31: 491–543, 1980.
Buschmann, C., Schrey, H.: Fluorescence induction kinetics of green and etiolated leaves by recording the complete in-vivo emission spectra.-Photosynth. Res. 1: 233–241, 1980.
Enami, I., Kitamura, M., Tomo, T., Isokawa, Y., Ohta, H., Katoh, S.: Is the primary cause of thermal inactivation of oxygen evolution in spinach PS II membranes release of the 33 extrinsic kDa protein or of Mn?-Biochim. biophys. Acta 1186: 52–58, 1994.
Fork, D.C., Satoh, K.: The control by state transitions of the distribution of excitation energy in photosynthesis.-Annu. Rev. Plant Physiol. 37: 335–361, 1986.
Franck, F., Juneau, P., Popovic, R.: Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature.-Biochim. biophys. Acta 1556: 239–246, 2002.
Georgieva, K., Lichtenthaler, H.K.: Photosynthetic activity and acclimation ability of pea plants to low and high temperature treatment as studied by means of chlorophyll fluorescence.-J. Plant Physiol. 155: 416–423, 1999.
Georgieva, K., Yordanov, I.: Temperature dependence of chlorophyll fluorescence parameters of pea seedlings.-J. Plant Physiol. 142: 151–155, 1993.
Georgieva, K., Yordanov, I., Tsonev, T.: Influence of low temperature treatment on the functional activity and acclimation ability of the photosynthetic apparatus of pea plants.-Compt. rend. bulg. Acad. Sci. 52: 71–74, 1992.
Gitelson, A.A., Buschmann, C., Lichtenthaler, H.K.: Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements.-J. Plant Physiol. 152: 283–296, 1998.
Govindjee: Chlorophyll a fluorescence: a bit of basics and history.-In: Papageorgiu, G.C., Govindjee (ed.): Chlorophyll a Fluorescence. A Signature of Photosynthesis. Pp. 1–42. Springer, Dordrecht 2004.
Havaux, M.: Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures.-Plant Cell Environ. 16: 461–467, 1993.
Havaux, M.: Temperature-dependent modulation of the photoinhibition-sensitivity of photosystem II in Solanum tuberosum leaves.-Plant Cell Physiol. 35: 757–766, 1994.
Havaux, M., Strasser, R.J.: Antagonistic effects of red and farred lights on the stability of photosystem II in pea leaves exposed to heat.-Photochem. Photobiol. 55: 621–624, 1992.
Janssen, L.H.J., Wams, H.E., van Hasselt, P.R.: Temperature dependence of chlorophyll fluorescence induction and photosynthesis in tomato as affected by temperature and light conditions during growth.-J. Plant Physiol. 139: 549–554, 1992.
Kocsányi, L., Haitz, M., Lichtenthaler, H.K.: Measurement of the laser-induced chlorophyll fluorescence kinetics using a fast acousto optic device.-In: Lichtenthaler, H.K. (ed.): Applications of Chlorophyll Fluorescence. Pp. 99–107. Kluwer Academic Publishers, Dordrecht-Boston-London 1988.
Krause, G.H., Weis, E.: Chlorophyll fluorescence and photosynthesis: The basics.-Annu. Rev. Plant Physiol. Plant mol. Biol. 42: 313–349, 1991.
Lichtenthaler, H.K.: Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown.-J. Plant Physiol. 131: 101–110, 1987a.
Lichtenthaler, H.K.: Chlorophylls and carotenoids-pigments of photosynthetic biomembranes.-In: Colowick, S.P., Kaplan, N.O. (ed.): Methods in Enzymology. Vol. 148. Pp. 350–382. Academic Press, San Diego-New York-Berkeley-Boston-London-Sydney-Tokyo-Toronto 1987b.
Lichtenthaler, H.K.: Vegetation stress: an introduction to the stress concept in plants.-J. Plant Physiol. 148: 4–14, 1996.
Lichtenthaler, H.K., Babani, F.: Light adaptation and senescence of the photosynthetic apparatus: changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity.-In: Papageorgiou, G.C., Govindjee (ed.): Chlorophyll a Fluorescence. A Signature of Photosynthesis. Pp. 713–736. Springer, Dordrecht 2004.
Lichtenthaler, H.K., Burkart, S., Schindler, C., Stober, F.: Changes in photosynthetic pigments and in vivo chlorophyll fluorescence parameters under photoinhibitory growth conditions.-Photosynthetica 27: 343–353, 1992.
Lichtenthaler, H.K., Buschmann, C.: Chlorophylls and carotenoids-Measurement and characterisation by UV-VIS.-Current Protocols in Food Analytical Chemistry (CPFA), (Supplement 1), F4.3.1-F 4.3.8. John Wiley, New York 2001.
Lichtenthaler, H.K., Buschmann, C., Knapp, M.: How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer.-Photosynthetica 43: 379–393, 2005a.
Lichtenthaler, H.K., Kuhn, G., Prenzel, U., Buschmann, C., Meier, D.: Adaptation of chloroplast-ultrastructure and of chlorophyll-protein levels to high-light and low-light growth conditions.-Z. Naturforsch. 37c: 464–475, 1982a.
Lichtenthaler H.K., Langsdorf, G., Lenk, S., Buschmann, C.: Chlorophyll fluorescence imaging of photosynthetic activity with the flash-lamp fluorescence imaging system.-Photosynthetica 43: 355–369, 2005b.
Lichtenthaler, H.K., Miehé, J.A.: Fluorescence imaging as a diagnostic tool for plant stress.-Trends Plant Sci. 2: 316–320, 1997.
Lichtenthaler, H.K., Prenzel, U., Kuhn, G.: Carotenoid composition of chlorophyll-carotenoid-proteins from radish chloroplasts.-Z. Naturforsch. 37c: 10–12, 1982b.
Lichtenthaler, H.K., Rinderle, U.: The role of chlorophyll fluorescence in the detection of stress conditions in plants.-CRC crit. Rev. anal. Chem. 19: S29–S85, 1988.
Maciejewska, U., Bauer, H.: Effects of cold acclimation on chlorophyll fluorescence in winter rape leaves.-Photosynthetica 28: 559–562, 1993.
Mamedov, M., Hayashi, H., Murata, N.: Effects of glycinebetaine and unsaturation of membrane lipids on heat stability of photosynthetic electron transport and phosphorylation reactions in Synechocystis PCC6803.-Biochim. biophys. Acta 1142: 1–5, 1993.
Nishiyama, Y., Kovacs, E., Lee, C.B., Hayashi, H., Watanabe, T., Murata, N.: Photosynthetic adaptation to high temperature associated with thylakoid membranes of Synechococcus PCC7002.-Plant Cell Physiol. 34: 337–343, 1993.
Öquist, G., Hurry, V.M., Huner, N.P.A.: The temperature dependence of the redox state of QA and susceptibility of photosynthesis to photoinhibition.-Plant Physiol. Biochem. 31: 683–691, 1993.
Papageorgiou, G.: Chlorophyll fluorescence: an intrinsic probe of photosynthesis.-In: Govindjee (ed.): Bioenergetics of Photosynthesis. Pp. 319–371. Academic Press, New York-San Francisco-London 1975.
Pfündel, E.: Estimating the contribution of Photosystem I to total leaf chlorophyll fluorescence.-Photosynth. Res. 56: 185–195, 1998.
Roháček, K.: Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual ralationships.-Photosynthetica 40: 13–29, 2002.
Sayed, O.H., Earnshaw, M.J., Emes, M.J.: Characterization of the heat-induced stimulation of photosystem-I-mediated electron transport.-Acta bot. neerl. 43: 137–143, 1994.
Schindler, C., Reith, P., Lichtenthaler, H.K.: Differential levels of carotenoids and decrease of zeaxanthin cycle performance during leaf development in a green and an aurea variety of tobacco.-J. Plant Physiol. 143: 500–507, 1994.
Schreiber, U., Schliwa, U., Bilger, W.: Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer.-Photosynth. Res. 10: 51–62, 1986.
Strasser, R.J., Schwarz, B., Bucher, J.: Simultane Messung der Chlorophyll Fluoreszenz-Kinetik bei verschiedenen Wellenlängen als rasches Verfahren zur Frühdiagnose von Immissionsbelastungen an Waldbäumen. Ozoneinwirkungen auf Buchen und Pappeln.-Eur. J. Forest Pathol. 17: 149–157, 1987.
Subhash, N., Mohanan, C.N., Mallia, R.J., Murlidharan, V.: Quantification of stress adaptation by laser-induced fluorescence spectroscopy of plants exposed to engine exhaust emission and drought.-Funct. Plant Biol. 31: 709–713, 2004.
Zhang, F., Zhang, L., Li, S.Y.: Effect of high temperature stress on leaf photosynthesis of citrus during blossom and young fruit stage.-Acta Horticult. sin. 22: 11–15, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Georgieva, K., Lichtenthaler, H.K. Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica 44, 569–578 (2006). https://doi.org/10.1007/s11099-006-0073-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11099-006-0073-y