Abstract
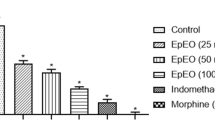
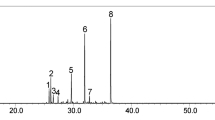
The analgesic and anti-inflammatory properties of Citrus aurantium L. blossoms essential oil (neroli) were investigated in mice and rats. The analgesic activity of neroli was assessed by acetic acid-induced writhing and Eddy’s hot plate methods, while acute and chronic anti-inflammatory effects were investigated by inflammatory paw edema in rat and the cotton pellet-induced granuloma tissue model, respectively. Mechanistic studies were conducted using L-nitro arginine methyl ester (L-NAME), an inhibitor of NO synthase. Neroli significantly decreased the number of acetic acid-induced writhes in mice compared to animals that received vehicle only. Also, it exhibited a central analgesic effect, as evidenced by a significant increase in reaction time in the hot plate method. The oil also significantly reduced carrageenan-induced paw edema in rats. The inhibitory activity of neroli (especially at 40 mg/kg) was found to be very close to the standard drug, diclofenac sodium (50 mg/kg). In cotton pellet-induced granuloma, neroli was effective regarding the transudate and granuloma formation amount. Neroli was analyzed by gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS) and twenty-three constituents, representing 91.0 % of the oil, were identified. The major components of neroli were characterized as linalool (28.5 %), linalyl acetate (19.6 %), nerolidol (9.1 %), E,E-farnesol (9.1 %), α-terpineol (4.9 %), and limonene (4.6 %), which might be responsible for these observed activities. The results suggest that neroli possesses biologically active constituent(s) that have significant activity against acute and especially chronic inflammation, and have central and peripheral antinociceptive effects which support the ethnomedicinal claims of the use of the plant in the management of pain and inflammation.



Similar content being viewed by others
References
Hadjiakhoondi A, Baligh N (2005) Practical guidance of medicinal plants. Islamic Azad University Scientific Publication Center, Tehran
Monajemi R, Oryan S, Haeri-Roohani A, Ghannadi A, Jafarian A (2005) Cytotoxic effects of essential oils of some Iranian Citrus peels. Iran J Pharm Res 4:183–187
Carvalho-Freitas MIR, Costa M (2002) Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol Pharm Bull 25:1629–1633
Tisserand R, Balacs T (1995) Essential oil safety: a guide for health care professionals. Churchill Livingstone, London, p 208
Azanchi T, Shafaroodi H, Asgarpanah J (2014) Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): involvment of the GABAergic system. Nat Prod Commun 9(11):1615–1618
Abbasnejad M, Keramat B, Esmaili Mahani S, Rezaeezade Roukerd M (2012) Effect of hydro-methanolic extract of sour orange flowers, Citrus aurantium, on pentylentetrazole induced seizure in male rats. J Babol Univ Med Sci 14:18–22
Akhlaghi M, Shabanian G, Rafieian-Kopaei M, Parvin N, Saadat M, Akhlaghi M (2011) Citrus aurantium blossom and preoperative anxiety. Braz J Anesthesiol 61:707–712
Swigar AA, Silverstein RM (1981) Monoterpenes. Aldrich Chemical Co., Milwaukee
Adams RP (1995) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Co., Carol Stream
Siegmund E, Cadmus R, Lu G (1957) A method for evaluating both non-narcotic and narcotic analgesics. Proc Soc Exp Biol Med 95:729–731
MacDonald AD, Woolfe G, Bergel F, Morrison AL, Rinderknecht H (1946) Analgesic action of pethidine derivatives and related compounds. Br J Pharmacol Chemother 1:4–14
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med 111:544–547
Winter CA, Porter CC (1957) Effect of alterations in side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J Am Pharm Assoc Am Pharm Assoc (Baltim) 46:515–519
Duarte IDG, Nakamura M, Ferreira SH (1988) Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21:341–343
Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–114
Durate IDG, Lorenzetti BB, Ferreira SH (1990) Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Eur J Pharmacol 186(2–3):289–293
Fidecka S, Lalewicz S (1997) Studies on the antinociceptive effects of sodium nitroprusside and molsidomine in mice. Pol J Pharmacol 49(6):395–400
Levy D, Strassman AM (2004) Modulation of dural nociceptor mechanosensitivity by the nitric oxide-cyclic GMP signaling cascade. J Neurophysiol 92(2):766–772
Prado WA, Schiavon VF, Cunha FQ (2002) Dual effect of local application of nitric oxide donors in a model of incision pain in rats. Eur J Pharmacol 441(1–2):57–65
Peana AT, D’Aquila PS, Chessa ML, Moretti MD, Serra G, Pippia P (2003) (−)-Linalool produces antinociception in two experimental models of pain. Eur J Pharmacol 460(1):37–41
do Amaral JF, Silva MI, Neto MR, Neto PF, Moura BA, de Melo CT, de Araújo FL, de Sousa DP, de Vasconcelos PF, de Vasconcelos SM, de Sousa FC (2007) Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol Pharm Bull 30(7):1217–1220
Quintans-Júnior LJ, Oliveira MG, Santana MF, Santana MT, Guimarães AG, Siqueira JS, De Sousa DP, Almeida RN (2011) α-Terpineol reduces nociceptive behavior in mice. Pharm Biol 49(6):583–586
Just MJ, Recio MC, Giner RM, Cuéllar MJ, Máñez S, Bilia AR, Ríos JL (1998) Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med 64:404–407
Swingle KF, Shideman FE (1972) Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J Pharmacol Exp Ther 183:226–234
Rivot JP, Montagne-Clavel J, Besson JM (2002) Subcutaneous formalin and intraplantar carrageenan increase nitric oxide release as measured by in vivo voltammetry in the spinal cord. Eur J Pain 6(1):25–34
Peana AT, D’Aquila PS, Panin F, Serra G, Pippia P, Moretti MD (2002) Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 9(8):721–726
Ueno A, Naraba H, Ikeda Y, Ushikubi F, Murata T, Narumiya S, Oh-ishi S (2000) Intrinsic prostacyclin contributes to exudation induced by bradykinin or carrageenin: a study on the paw edema induced in IP-receptor-deficient mice. Life Sci 66:PL155–PL160
Acknowledgments
Support from the Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khodabakhsh, P., Shafaroodi, H. & Asgarpanah, J. Analgesic and anti-inflammatory activities of Citrus aurantium L. blossoms essential oil (neroli): involvement of the nitric oxide/cyclic-guanosine monophosphate pathway. J Nat Med 69, 324–331 (2015). https://doi.org/10.1007/s11418-015-0896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-015-0896-6