Abstract
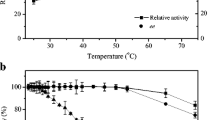
Recombinant Escherichia coli cells expressing Alcaligenes sp. nitrilase were simply immobilized by direct cross-linking using glutaraldehyde. About 85 % of the total nitrilase activity was recovered under the optimal cross-linking conditions. The thermal stabilities of the cross-linked cells measured at 30, 40 and 50 °C were 4.5-, 5.3-, and 5.1-fold those of the free cells, respectively. The concentration of (R)-(−)-mandelic acid reached 280 mM after merely 2 h transformation with the immobilized cells using 300 mM mandelonitrile as substrate, affording an extremely high productivity of 510.7 g L−1 d−1. In addition, operational stability of the immobilized cells was obviously superior to that of free cells, without significant activity loss after 15 cycles of batch reactions or 8 cycles of repeated fed-batch reactions. Therefore, the easy preparation and robust characteristics of the immobilized biocatalyst make it a very promising biocatalyst for high-performance and low-cost production of optically pure (R)-(−)-mandelic acid.







Similar content being viewed by others
References
Gong JS, Lu ZM, Li H, Shi JS, Zhou ZM, Xu ZH (2012) Nitrilases in nitrile biocatalysis: recent progress and forthcoming research. Microb Cell Fact 11:142–159
Groger H (2001) Enzymatic routes to enantiomerically pure aromatic α-hydroxy carboxylic acids: a further example for the diversity of biocatalysis. Adv Synth Catal 343:547–558
Wang MX (2005) Enantioselective biotransformations of nitriles in organic synthesis. Top Catal 35:117–130
Wang MX (2009) Progress of enantioselective nitrile biotransformations in organic synthesis. Chimia 63:331–333
Zhu D, Ankati H, Mukherjee C, Yang Y, Biehl ER, Hua L (2007) Asymmetric reduction of β-ketonitriles with a recombinant carbonyl reductase and enzymatic transformation to optically pure β-hydroxy carboxylic acids. Org Lett 9:2561–2563
Ju X, Yu HL, Pan J, Wei DZ, Xu JH (2010) Bioproduction of chiral mandelate by enantioselective deacylation of α-acetoxyphenylacetic acid using whole cells of newly isolated Pseudomonas sp. ECU1011. Appl Microbiol Biotechnol 86:83–91
Golynskiy MV, Seelig B (2010) De Novo enzymes: from computational design to mRNA display. Trends Biotechnol 28:340–345
He YC, Xu JH, Pan J, Ouyang LM, Xu Y (2008) Preparation of (R)-(−)-mandelic acid and its derivatives from racemates by enantioselective degradation with a newly isolated bacterial strain Alcaligenes sp. ECU0401. Bioprocess Biosyst Eng 31:445–451
Oda S, Kikuchi Y, Nanishi Y (1992) Synthesis of optically active mandelic acid via microbial oxidation of racemic 1-phenyl-1,2-ethanediol. Biosci Biotechnol Biochem 56:1216–1220
Patterson MAK, Szajewski RP, Whitesides GM (1981) Enzymatic conversion of alpha-keto aldehydes to optically active alpha hydroxy acids using glyoxalase I and II. J Org Chem 46:4682–4685
Xiao MT, Huang YY, Shi XA, Guo YH (2005) Bioreduction of phenylglyoxylic acid to R-(−)-mandelic acid by Saccharomyces cerevisiae FD11b. Enzyme Microb Technol 37:589–596
Yadav GD, Sivakumar P (2004) Enzyme-catalysed optical resolution of mandelic acid via RS(∓)-methyl mandelate in non-aqueous media. Biochem Eng J 19:101–107
Yamamoto K, Oishi K, Fujimatsu I, Komatsu KI (1991) Production of R-(−)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl Environ Microbiol 57:3028–3032
Banerjee A, Kaul P, Banerjee UC (2006) Enhancing the catalytic potential of nitrilase from Pseudomonas putida for stereoselective nitrile hydrolysis. Appl Microbiol Biotechnol 72:77–87
Kaul P, Banerjee A, Mayilraj S, Banerjee UC (2004) Screening for enantioselective nitrilases: kinetic resolution of racemic mandelonitrile to (R)-(−)-mandelic acid by new bacterial isolates. Tetrahedron Asymmetry 15:207–211
Kaul P, Banerjee A, Banerjee UC (2006) Stereoselective nitrile hydrolysis by immobilized whole-cell biocatalyst. Biomacromolecules 7:1536–1541
Singh R, Banerjee A, Kaul P, Barse B, Banerjee UC (2005) Release of an enantioselective nitrilase from Alcaligenes faecalis MTCC 126: a comparative study. Bioprocess Biosyst Eng 27:415–424
Wang H, Sun H, Wei D (2013) Discovery and characterization of a highly efficient enantioselective mandelonitrile hydrolase from Burkholderia cenocepacia J2315 by phylogeny-based enzymatic substrate specificity prediction. BMC Biotechnol 13:14–24
Xue YP, Liu ZQ, Xu M, Wang YJ, Zheng YG, Shen YC (2010) Enhanced biotransformation of (R, S)-mandelonitrile to (R)-(−)-mandelic acid with in situ production removal by addition of resin. Biochem Eng J 53:143–149
Xue YP, Xu SZ, Liu ZQ, Zheng YG, Shen YC (2011) Enantioselective biocatalytic hydrolysis of (R, S)-mandelonitrile for production of (R)-(−)-mandelic acid by a newly isolated mutant strain. J In Microbiol Biotechnol 38:337–345
He YC, Xu JH, Xu Y, Ouyang LM, Pan J (2007) Biocatalytic synthesis of (R)-(−)-mandelic acid from racemic mandelonitrile by a newly isolated nitrilase-producer Alcaligenes sp. ECU0401. Chin Chem Lett 18:677–680
He YC, Zhang ZJ, Xu JH, Liu YY (2010) Biocatalytic synthesis of (R)-(−)-mandelic acid from racemic mandelonitrile by cetyltrimethylammonium bromidepermeabilized cells of Alcaligenes faecalis ECU0401. J Ind Microbiol Biotechnol 37:741–750
Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY, Imanaka T (2010) Efficient production of (R)-(–)-mandelic acid with highly substrate/product tolerant and enantioselective nitrilase of recombinant Alcaligenes sp. Process Biochem 45:887–891
Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY (2011) Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. ECU0401 and its potential for (R)-(−)-mandelic acid production. Bioprocess Biosyst Eng 34:315–322
Zhang ZJ, Pan J, Liu JF, Xu JH, He YC, Liu YY (2011) Significant enhancement of (R)-mandelic acid production by relieving substrate inhibition of recombinant nitrilase in toluene-water biphasic system. J Biotechnol 152:24–29
Zheng GW, Yu HL, Li CX, Pan J, Xu JH (2011) Immobilization of Bacillus subtilis esterase by simple cross-linking for enzymatic resolution of dl-menthyl acetate. J Mol Catal B Enzym 70:138–143
Mylerova V, Martinkova L (2003) Synthetic applications of nitrile-converting enzymes. Curr Org Chem 7:1279–1295
Roach PCJ, Ramsden DK, Hughes J, Williams P (2004) Biocatalytic scrubbing of gaseous acrylonitrile using Rhodococcus ruber immobilized in synthetic silicone polymer (ImmobaSil) rings. Biotechnol Bioeng 85:450–455
Kabaivanova L, Dobreva E, Dimitrov P, Emanuilova E (2005) Immobilization of cells with nitrilase activity from a thermophilic bacterial strain. J Ind Microbiol Biotechnol 32:7–11
Vejvoda V, Kaplan O, Benzouska K, Martinkova L (2006) Mild hydrolysis of nitriles by the immobilized nitrilase from Aspergillus niger K10. J Mol Catal B Enzym 39:55–58
Kaul P, Stolz A, Banerjee UC (2007) Cross-linked amorphous nitrilase aggregates for enantioselective nitrile hydrolysis. Adv Synth Catal 349:2167–2176
Kumar S, Mohan U, Kamble AL, Powar S, Banerjee UC (2010) Cross-linked enzyme aggregates of recombinant Pseudomonas putida nitrilase for enantioselective nitrile hydrolysis. Bioresour Technol 101:6856–6858
Sheldon RA (2011) Cross-linked enzyme aggregates as industrial biocatalysts. Org Process Res Dev 15:213–233
Mateo C, Fernandes B, van Rantwijk F, Stolz A, Sheldon RA (2006) Stabilisation of oxygen-labile nitrilases via co-aggregation with poly(ethyleneimine). J Mol Catal B Enzym 38:154–157
Mateo C, Palomo JM, van Langen LM, van Rantwijk F, Sheldon RA (2004) A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol Bioeng 86:273–276
Liu JF, Zhang ZJ, Li AT, Pan J, Xu JH (2011) Significantly enhanced production of recombinant nitrilase by optimization of culture conditions and glycerol feeding. Appl Microbiol Biotechnol 89:665–672
Xue YP, Xu M, Chen HS, Liu ZQ, Wang YJ, Zheng YG (2013) A novel integrated bioprocess for efficient production of (R)-(−)-mandelic acid with immobilized Alcaligenes faecalis ZJUTB10. Org Process Res Dev 17:213–220
Pawar SV, Meena VS, Kaushik S, Kamble A, Kumar S, Chisti Y, Banerjee UC (2012) Stereo-selective conversion of mandelonitrile to (R)-(−)-mandelic acid using immobilized cells of recombinant Escherichia coli. 3. Biotech 2:319–326
Acknowledgments
This work was financially supported by the National Science Foundation of China (Nos. 21276082 & 31200050), Ministry of Science and Technology, P. R. China (Nos. 2011CB710800, 2011AA02A210 & 2012AA022201), Shanghai Commission of Science and Technology (No. 11431921600) and the Fundamental Research Funds for the Central Universities (No. 222201314016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, ZJ., Pan, J., Li, CX. et al. Efficient production of (R)-(−)-mandelic acid using glutaraldehyde cross-linked Escherichia coli cells expressing Alcaligenes sp. nitrilase. Bioprocess Biosyst Eng 37, 1241–1248 (2014). https://doi.org/10.1007/s00449-013-1096-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1096-y