Abstract
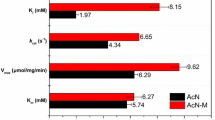
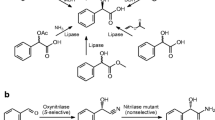
(R)-(−)-Mandelic acid (R-MA) is an important intermediate with broad uses. Recently, R-MA production using nitrilase has been gaining more and more attention due to its higher productivity and enantioselectivity. In this work, a new bacterium WT10, which exhibited favorable nitrilase activity and excellent enantioselectivity for production of R-MA by enantioselective biocatalytic hydrolysis of (R,S)-mandelonitrile, was isolated and identified as a strain of Alcaligenes faecalis. In order to improve its nitrilase activity for industrial application, the wild-type strain WT10 was further subjected to mutagenesis using a combined LiCl–ultraviolet irradiation and low energy N+ ion beams implantation technique. A valuable mutant strain A. faecalis ZJUTB10 was obtained. The nitrilase specific activity of the mutant strain was greatly improved up to 350.8 U g−1, in comparison with wild-type strain WT10 of 53.09 U g−1. The reaction conditions for R-MA production by mutant strain A. faecalis ZJUTB10 were also optimized. Nitrilase activity in mutant strain showed a broad pH optimum at pH 7.7–8.5. The optimal temperature was 35°C. The highest production rate reached 9.3 mmol h−1 g−1. The results showed that mutant strain A. faecalis ZJUTB10 was a new candidate for efficient R-MA production from (R,S)-mandelonitrile and could potentially be used in industrial production.









Similar content being viewed by others
References
Banerjee A, Kaul P, Banerjee UC (2006) Enhancing the catalytic potential of nitrilase from Pseudomonas putida for stereoselective nitrile hydrolysis. Appl Microbiol Biotechnol 72:77–87
Banerjee A, Kaul P, Sharma R, Banerjee UC (2003) A high-throughput amenable colorimetric assay for enantioselective screening of nitrilase-producing microorganisms using pH sensitive indicators. J Biomol Screen 8:559–565
Chen J, Zheng RC, Zheng YG, Shen YC (2009) Microbial transformation of nitriles to high-value acids or amides. Adv Biochem Eng Biotechnol 113:33–77
Chen J, Zheng YG, Shen YC (2008) Biotransformation of p-methoxyphenylacetonitrile into p-methoxyphenylacetic acid by resting cells of Bacillus subtilis. Biotechnol Appl Biochem 50:147–153
Chung CT, Niemela SL, Miller RH (1989) One-step preparation of competent Escherichia coli—transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA 86:2172–2175
He YC, Xu JH, Xu Y, Ouyang LM, Pan J (2007) Biocatalytic synthesis of (R)-(−)-mandelic acid from racemic mandelonitrile by a newly isolated nitrilase-producer Alcaligenes sp ECU0401. Chin Chem Lett 18:677–680
Kaul P, Banerjee A, Banerjee UC (2006) Stereoselective nitrile hydrolysis by immobilized whole-cell biocatalyst. Biomacromolecules 7:1536–1541
Kaul P, Banerjee A, Mayilraj S, Banerjee UC (2004) Screening for enantioselective nitrilases: kinetic resolution of racemic mandelonitrile to (R)-(−)-mandelic acid by new bacterial isolates. Tetrahedron Asymmetry 15:207–211
Liu ZQ, Zhang JF, Zheng YG, Shen YC (2008) Improvement of astaxanthin production by a newly isolated Phaffia rhodozyma mutant with low-energy ion beam implantation. J Appl Microbiol 104:861–872
Miyamoto K, Ohta H (1992) Enantioselective oxidation of mandelic acid using a phenylmalonate metabolizing pathway of a soil bacterium Alcaligenes bronchisepticus Ku1201. Biotechnol Lett 14:363–366
Oda S, Kikuchi Y, Nanishi Y (1992) Synthesis of optically active mandelic acid via microbial oxidation of racemic 1-phenyl-1, 2-ethanediol. Biosci Biotechnol Biochem 56:1216–1220
Patterson MAK, Szajewski RP, Whitesides GM (1981) Enzymic conversion of alpha-keto aldehydes to optically active alpha-hydroxy acids using glyoxalase I and II. J Org Chem 46:4682–4685
Singh R, Banerjee A, Kaul P, Barse B, Banerjee UC (2005) Release of an enantioselective nitrilase from Alcaligenes faecalis MTCC 126: a comparative study. Bioprocess Biosyst Eng 27:415–424
Singh R, Sharma R, Tewari N, Geetanjali, Rawat DS (2006) Nitrilase and its application as a ‘green’ catalyst. Chem Biodivers 3:1279–1287
Tang KW, Yi JM, Huang KL, Zhang GL (2009) Biphasic recognition chiral extraction: a novel method for separation of mandelic acid enantiomers. Chirality 21:390–395
Wang MX (2005) Enantioselective biotransformations of nitriles in organic synthesis. Top Catal 35:117–130
Xiao MT, Huang YY, Shi XA, Guo YH (2005) Bioreduction of phenylglyoxylic acid to R-(−)-mandelic acid by Saccharomyces cerevisiae FD11b. Enzyme Microb Technol 37:589–596
Xiao MT, Huang YY, Ye J, Guo YH (2008) Study on the kinetic characteristics of the asymmetric production of R-(−)-mandelic acid with immobilized Saccharomyces cerevisiae FD11b. Biochem Eng J 39:311–318
Yadav GD, Sivakumar P (2004) Enzyme-catalysed optical resolution of mandelic acid via RS(∓)-methyl mandelate in non-aqueous media. Biochem Eng J 19:101–107
Yamamoto K, Oishi K, Fujimatsu I, Komatsu KI (1991) Production of R-(−)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl Environ Microbiol 57:3028–3032
Yu Z (2000) Ion beam application in genetic modification. IEEE Trans Plasma Sci 28:128–132
Yu Z, Deng J, He J, Huo Y, Wu Y, Wang X, Lui G (1991) Mutation breeding by ion implantation. Nucl Instrum Methods Phys Res B59(60):705–708
Acknowledgments
This work was supported by the Fund of the National High Technology Research and Development Program of China (863 Program) (No. 2009AA02Z203), the Major Basic Research Development Program of China (973 Project) (No. 2009CB724704), and Natural Science Foundation of Zhejiang Province (No. Z4090612).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, YP., Xu, SZ., Liu, ZQ. et al. Enantioselective biocatalytic hydrolysis of (R,S)-mandelonitrile for production of (R)-(−)-mandelic acid by a newly isolated mutant strain. J Ind Microbiol Biotechnol 38, 337–345 (2011). https://doi.org/10.1007/s10295-010-0778-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0778-6