Abstract
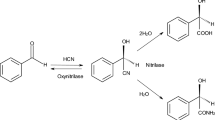
An enantioselective mandelate-degrading bacterium, Alcaligenes sp. ECU0401, was newly isolated from soil. By fed-batch culture, (R)-(−)-mandelic acid was successfully prepared in a 5-L fermenter with 32.8% isolated yield and >99.9% enantiomeric excesses (e.e.) from totally 3.04% (w/v) of racemic mandelic acid after 99 h of biotransformation. The optimal reaction pH and temperature were 6.5 and 30 °C, respectively. Using the resting cell as a biocatalyst for asymmetric degradation of racemic mandelic acid and chloro-substituted derivatives thereof, (R)-(−)-mandelic acid, (R)-(−)-o-chloromandelic acid, (S)-(+)-m-chloromandelic acid and (S)-(+)-p-chloromandelic acid were recovered with high analytic yields and excellent enantiomeric excesses (e.e. > 99.9%). (R)-(−)-Mandelic acid could also be obtained after 12 h of biotransformation with 41.5% isolated yield and >99.9% e.e.








Similar content being viewed by others
References
De Santis G, Zhu ZL, Greenberg WA, Wong K, Chaplin J, Hanson SR, Farwell B, Nicholson LW, Rand CL, Weiner DP, Robertson DE, Burk MJ (2002) An enzyme library approach to biocatalysis: development of nitrilases for enantioselective production of carboxylic acid derivatives. J Am Chem Soc 124:9024–9025
Yamamoto K, Fujimatsu I, Komatsu K (1992) Purification and characterization of the nitrilase from Alcaligenes faecalis ATCC 8750 responsible for enantioselective hydrolysis of mandelonitrile. J Ferment Bioeng 73:425–430
Kaul P, Banerjee A, Mayilraj S, Banerjee UC (2004) Screening for enantioselective nitrilases: kinetic resolution of racemic mandelonitrile to (R)-(−)-mandelic acid by new bacterial isolates. Tetrahedron Asymmetry 15:207–211
Baar MR, Cerrone-Szakal AL (2005) Enantiomeric resolution of (±)-mandelic acid by (1R,2S)-(−)-ephedrine: an organic chemistry laboratory experiment illustrating stereoisomerism. J Chem Educ 82:1040–1042
Rong F, Feng XG, Yuan CW, Fu DG, Li P (2006) Chiral separation of mandelic acid and its derivatives by thin layer chromatography using molecularly imprinted stationary phases. J Liq Chromatogr Relat Technol 29:2593–2602
Sarhan A, El-Zahab MA (1987) Racemic resolution of mandelic acid on polymers with chiral cavities, 2. Enzyme-analogue stereospecific conversion of configuration. Die Makromol Chem Rapid Commun 8:555–561
Lorenz H, Polenske D, Seidel-Morgenstern A (2006) Morgenstern application of preferential crystallization to resolve racemic compounds in a hybrid process. Chirality 18:828–840
Bauza R, Rios A, Valcarcel M (2004) Enantioselective supercritical fluid extraction from racemic mixtures by use of chiral selectors. Sep Sci Technol 39:459–478
Elliot JD, Choi VFM, Johnson WS (1983) Asymmetric synthesis via acetal templates. 5. Reactions with cyanotrimethylsilane. Enantioselective preparation of cyanohydrins and derivatives. J Org Chem 48:2294–2295
Yamamoto KZ, Oishi K, Fujimashu I, Komatsu K (1991) Production of R-(−)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl Environ Microbiol 57:3028–3032
Yamazaki Y, Maeda H (1986) (R)-(−)-Mandelic acid and other 2-hydroxycarboxylic acids: screening for the enzyme, and its purification, characterization and use. Agric Biol Chem 50:2621–2631
Fernandez-Lorente G, Fernández-Lafuente R, Palomo JM, Mateo C, Bastida A, Coca J, Haramboure T, Hernández-Justiz O, Terreni M, Guisán JM (2001) Biocatalyst engineering exerts a dramatic effect on selectivity of hydrolysis catalyzed by immobilized lipases in aqueous medium. J Mol Catal B Enzym 11:649–656
Strauss UT, Kandelbauer A, Faber K (2000) Stabilization and activity-enhancement of mandelate racemase from Pseudomonas putida ATCC 12336 by immobilization. Biotechnol Lett 22:515–520
Shinobu O, Yutaka K, Yasushi N (1992) Synthesis of optically active mandelic acid via microbial oxidation of racemic 1-phenyl-1,2-ethanediol. Biosci Biotechnol Biochem 56:1216–1220
Patterson MAK, Szajewski RP, Whitesides GM (1981) Enzymatic conversion of α-keto aldehydes to optically active α-hydroxy acids using glyoxalase I and II. J Org Chem 46:4682–4685
Takahashi E, Nakamichi K, Furui M, Mori T (1995) R-(−)-Mandelic acid production from racemic mandelic acids by Pseudomonas polycolor with asymmetric degrading activity. J Ferment Bioeng 79:439–442
Huang HR, Xu JH (2006) Preparation of (S)-mandelic acid from racemate using growing cells of Pseudomonas putida ECU1009 with (R)-mandelate degradation activity. Biochem Eng J 30:11–15
Gihani MTE, Williams JMJ (1999) Dynamic kinetic resolution. Curr Opin Chem Biol 3:11–15
Tsuchiya S, Miyamoto K, Ohta H (1992) Highly efficient conversion of (±)-mandelic acid to its (R)-(−)-enantiomer by combination of enzyme-mediated oxidation and reduction. Biotechnol Lett 14:1137–1142
Jamaluddin M, Rao PVS, Vaidyanathan CS (1970) Involvement of the protocatechulate pathway in the metabolism of mandelic acid by Aspergillus niger. J Bacteriol 101:786–793
Takahashi E, Nakamichi K, Furui M, Mori T (1995) R-(−)-Mandelic acid production from racemic mandelic acids using Pseudomonas polycolor IFO 3918 and Micrococcus freudenreichii FERM-P 13221. J Ferment Bioeng 3:247–250
He YC, Xu JH, Xu Y, Ouyang LM, Pan J (2007) Biocatalytic synthesis of (R)-mandelic acid from racemic mandelonitrile by a newly isolated nitrilase-producer Alcaligenes sp. ECU0401. Chin Chem Lett 18:677–680
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Huang HR, Xu JH, Xu Y, Pan J, Liu X (2005) Preparation of (S)-mandelic acids by enantioselective degradation of racemates with a new isolate Pseudomonas putida ECU1009. Tetrahedron Asymmetry 16:2113–2117
Liese A, Seelbach K, Wandrey C (2000) Process. In: Industrial Biotransformations, Wiley-VCH, Weinheim, pp 93–392
Faber K (1997) Biocatalytical applications. In: Biotransformations in Organic Chemistry. Springer, Berlin, pp 29–331
Gröger H (2001) Enzymatic routes to enantiomerically pure aromatic α-hydroxy carboxylic acids: a further example for the diversity of biocatalysis. Adv Synth Catal 343:547–558
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (grant no. 20506037) and the Ministry of Education, P. R. China (grant no. 20050251011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, YC., Xu, JH., Pan, J. et al. Preparation of (R)-(−)-mandelic acid and its derivatives from racemates by enantioselective degradation with a newly isolated bacterial strain Alcaligenes sp. ECU0401. Bioprocess Biosyst Eng 31, 445–451 (2008). https://doi.org/10.1007/s00449-007-0181-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0181-5