Abstract
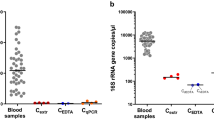
Bacterial endotoxins have been associated with chronic inflammation and the development and progression of diabetic nephropathy. We hypothesized that subjects with high serum lipopolysaccharide activity also carry remains of bacterial DNA in their system. Serum-derived bacterial DNA clones were isolated and identified from 10 healthy controls and 14 patients with type 1 diabetes (T1D) using universal primers targeted to bacterial 16S rDNA. A total of 240 clones representing 35 unique bacterial species were isolated and identified. A significant proportion of the isolated bacteria could be assigned to our living environment. Proteobacteria was by far the most prevalent phylum among the samples. Notably, the patients had significantly higher frequencies of Stenotrophomonas maltophilia clones in their sera compared to the healthy controls. Real-time PCR analysis of S. maltophilia and Pseudomonas aeruginosa flagellin gene copy number in the human leukocyte DNA fraction revealed that the overall Pseudomonal bacterial load was higher in older patients with T1D. Serum IgA- and IgG-antibody levels against Pseudomonal bacteria Delftia acidovorans, P. aeruginosa, and S. maltophilia were also determined in 200 healthy controls and 200 patients with T1D. The patients had significantly higher serum levels of IgA antibodies against all three Pseudomonal bacteria. Additionally, the IgA antibodies against Pseudomonal bacteria correlated significantly with serum C-reactive protein. These findings indicate that recurrent or chronic Pseudomonal exposure may increase susceptibility to chronic inflammation in patients with T1D.


Similar content being viewed by others
References
Llaurado G, Gallart L, Tirado R et al (2012) Insulin resistance, low-grade inflammation and type 1 diabetes mellitus. Acta Diabetol 49:33–39
Fadini BG, Marcuzzo G, Marescotti MC, Kreutzenberg S, Avogaro A (2012) Elevated white blood cell count is associated with prevalence and development of the metabolic syndrome and its components in the general population. Acta Diabetol [Epub ahead of print]
Fornoni A, Ijaz A, Tejada T, Lenz O (2008) Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4:10–17
Saraheimo M, Teppo A-M, Forsblom C et al (2003) Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46:1402–1407
Muller LM, Gorter KJ, Hak E et al (2005) Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 41:281–288
Benfield T, Jensen JS, Nordestgaard BG (2007) Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia 50:549–554
Gornik I, Gornik O, Gasparovic V (2007) HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Res Clin Pract 77:120–125
Kornum JB, Thomsen RW, Riis A et al (2008) Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31:1541–1545
Lassenius MI, Pietiläinen KH, Kaartinen K et al (2011) Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34:1809–1815
Nymark M, Pussinen PJ, Tuomainen AM et al (2009) Serum lipopolysaccharide activity is associated with the progression of kidney disease in Finnish patients with type 1 diabetes. Diabetes Care 32:1689–1693
Pussinen PJ, Havulinna AS, Lehto M et al (2011) Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34:392–397
Cani PD, Amar J, Iglesias MA et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772
Amar J, Burcelin R, Ruidavets JB et al (2008) Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87:1219–1223
Laugerette F, Vors C, Geloen A et al (2011) Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 22:53–59
McGuckin MA, Eri R, Simms LA et al (2009) Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis 15:100–113
Secondulfo M, Iafusco D, Carratu R et al (2004) Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis 36:35–45
Kotilainen P, Jalava J, Meurman O et al (1998) Diagnosis of meningococcal meningitis by broad-range bacterial PCR with cerebrospinal fluid. J Clin Microbiol 36:2205–2209
Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G et al (2002) Multiserotype enzyme-linked immunosorbent assay as a diagnostic aid for periodontitis in large-scale studies. J Clin Microbiol 40:512–518
Tamura K, Peterson D, Peterson N et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F et al (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Felsenstein J (2005) PHYLIP (phylogeny interference package) version 3.6. Distributed by the Author, Department of Genome Sciences, University of Washington, Seattle
Marques da Silva R, Da Caugant, Eribe ERK et al (2006) Bacterial diversity in aortic aneurysms determined by 16S ribosomal RNA gene analysis. J Vasc Surg 44:1055–1060
Moriyama K, Ando C, Tashiro K et al (2008) Polymerase chain reaction detection of bacterial 16S rRNA gene in human blood. Microbiol Immunol 52:375–382
Nelson DE, Van Der Pol B, Dong Q et al (2010) Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS ONE 5:E14116
Renko J, Lepp PW, Oksala N et al (2008) Bacterial signatures in atherosclerotic lesions represent human commensals and pathogens. Atherosclerosis 201:192–197
Siala M, Gdoura R, Fourati H et al (2009) Broad-range PCR, cloning and sequencing of the full 16S rRNA gene for detection of bacterial DNA in synovial fluid samples of Tunisian patients with reactive and undifferentiated arthritis. Arthritis Res Ther 11:R102
Mariat D, Firmesse O, Levenez F et al (2009) The firmicutes/bacteroides ratio of the human microbiota changes with age. BMC Microbiol 9:123
Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J (2011) Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol 48:257–273
Qin J, Li R, Raes J et al (2010) A human gut microbial gene catalogue established by metagenomic sequecing. Nature 464:59–65
Cani PD, Bibiloni R, Knauf C et al (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481
Amar J, Chabo C, Waget A et al (2011) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3:559–572
Bahrani-Mougeot FK, Paster BJ, Coleman S et al (2008) Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol 46:2129–2132
Geerts SO, Nys M, De Mol P et al (2002) Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol 73:73–78
Lockhart PB, Brennan MT, Sasser HC et al (2008) Bacteremia associated with toothbrushing and dental extraction. Circulation 117:3118–3125
Lucas VS, Gafan G, Dewhurst S, Roberts GJ (2008) Prevalence, intensity and nature of bacteraemia after toothbrushing. J Dent 36:481–487
Knuuttila M, Suominen-Taipale L (2008) Periodontal status. In: Suominen-Taipale L, Nordblad A, Vehkalahti M, Aromaa A (eds) Oral health in the Finnish adult population. Publications of the National Public Health Institute B25, Helsinki, pp 49–53
Aspriello SD, Zizzi A, Tirabassi G et al (2011) Diabetes mellitus-associated periodontitis: differences between type 1 and type 2 diabetes mellitus. J Periodontal Res 46:164–169
Choi YH, McKeown RE, Mayer-Davis EJ et al (2011) Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care 34:381–386
Kshirsagar AV, Offenbacher S, Moss KL et al (2007) Antibodies to periodontal organisms are associated with decreased kidney function. The dental atherosclerosis risk in communities study. Blood Purif 25:125–132
Shultis WA, Weil EJ, Looker HC et al (2007) Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care 30:306–311
Larsen N, Vogensen FK, van den Berg FWJ et al (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5:e9085
Qureshi A, Mooney L, Denton M, Kerr KG (2005) Stenotrophomonas maltophilia in salad. Emerg Infect Dis 11:1157–1158
Brooke JS (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41
Driscoll JA, Brody SL, Kollef MH (2007) The epidemiology, pathogenesis and treament of Pseudomonas aeruginosa infections. Drugs 67:351368
Talon D, Bailly P, Leprat R et al (1994) Typing of hospital strains of Xanthomonas maltophilia by pulsed-field gel electrophoresis. J Hosp Infect 27:209–217
Spicuzza L, Sciuto C, Vitaliti G et al (2009) Emerging pathogens in cystic fibrosis: ten years of follow-up in a cohort of patients. Eur J Clin Microbiol Infect Dis 28:191–195
Conti S, dos Santos SSF, Koga-Ito CY, Jorge AOC (2009) Enterobacteriaceae and pseudomonadaceae on the dorsum of the human tongue. J Appl Oral Sci 17:375–380
Leung WK, Yau JYY, Cheung BPK et al (2003) Oral colonisation by aerobic and facultatively anaerobic gram-negative rods and yeast in Tibetans living in Lhasa. Arch Oral Biol 48:117–123
Senpuku H, Sogame A, Inoshita E et al (2003) Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology 49:301–309
Tada A, Senpuku H, Motozawa Y et al (2006) Association between commensal bacteria and opportunistic pathogens in the dental plaque of elderly individuals. Clin Microbiol Infect 12:776–781
Wendel M, Paul R, Heller AR (2007) Lipoproteins in inflammation and sepsis. II. Clinical aspects. Intensive Care Med 33:25–35
Zhang H-Y, Sun S-H, Guo Y-J et al (2005) Tissue distribution of a plasmid DNA containing epitopes of foot-and-mouth disease virus in mice. Vaccine 23:5632–5640
Mercer DK, Scott KP, Bruce-Johnson WA et al (1999) Fate of free DNA and transformation of the oral bacterium Streptococcus gordonii DL1 by plasmid DNA in human saliva. Appl Environ Microbiol 65:6–10
Tamkovich SN, Vlasov VV, Laktionov PP (2008) Circulating deoxyribonucleic acids in blood and their using in medical diagnostics. Mol Biol (Mosk) 42:12–23
Koren O, Spor A, Felin J et al (2011) Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA 108:4592–4598
Ott SJ, Mokhtari NEE, Musfeldt M et al (2006) Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 113:929–937
Watt S, Aesch B, Lanotte P et al (2003) Viral and bacterial DNA in carotid atherosclerotic lesions. Eur J Clin Microbiol Infect Dis 22:99–105
Amar J, Serino M, Lange C et al (2011) Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia 54:3055–3061
Acknowledgments
The study was supported by Folkhälsan Research Foundation (P.-H. G.), Wilhelm and Else Stockmann Foundation (P.-H. G., L. P., C. F., M. L.), Diabetes Research Foundation (M. L.), The Novo Nordisk Foundation (M. L.), and the Sigrid Juselius Foundation (P. J. P.). Part of this study was presented in abstract form at the 45th Annual Meeting of the Scandinavian Society for the Study of Diabetes (2010), Malmö, Sweden. Laboratory technicians and nurses R. Keva, M. Parkkonen, A.-R. Salonen, A. Sandelin, T. Soppela and J. Tuomikangas at the Folkhälsan Institute of Genetics are acknowledged for their skillful technical assistance. We also acknowledge the physicians and nurses at each study center (Supplementary file 5).
Conflict of interest
No potential conflicts of interest relevant to this paper were reported.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Massimo Federici.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peräneva, L., Fogarty, C.L., Pussinen, P.J. et al. Systemic exposure to Pseudomonal bacteria: a potential link between type 1 diabetes and chronic inflammation. Acta Diabetol 50, 351–361 (2013). https://doi.org/10.1007/s00592-012-0421-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-012-0421-2