Abstract
Onychomycosis is the most common fungal infection of the nail affecting the skin under the fingertips and the toes. Currently, available therapy for onychomycosis includes oral and topical therapies, either alone or in combination. Oral antifungal medication has been associated with poor drug bioavailability and potential gastrointestinal and systemic side effects. The objective of this study was to prepare and evaluate the luliconazole nail lacquer (LCZ-NL) for the effective treatment of onychomycosis. In the current work, LCZ-NL was formulated in combination with penetration enhancers to overcome poor penetration. A 32 full factorial formulation design of experiment (DOE) was applied for optimization of batches with consideration of dependent (drying time, viscosity, and rate of drug diffusion) and independent (solvent ratio and film former ratio) variables. The optimized formulation was selected based on drying time, viscosity, and rate of drug diffusion. The optimized formulation was further evaluated for % non-volatile content assay, smoothness of flow, water resistance, drug content, scanning electron microscope (SEM), atomic force microscope (AFM), X-ray diffraction (XRD), differential scanning calorimetry (DSC), in vitro drug release, ex vivo transungual permeation, antifungal efficacy, and stability study. The optimized LCZ-NL contained 70:30 solvent ratio and 1:1 film former ratio and was found to have ~ 1.79-fold higher rate of drug diffusion in comparison with LULY™. DSC and XRD studies confirmed that luliconazole retains its crystalline property in the prepared formulation. Antifungal study against Trichophyton spp. showed that LCZ-NL has comparatively higher growth inhibition than LULY™. Hence, developed LCZ-NL can be a promising topical drug delivery system for treating onychomycosis.
Graphical abstract










Similar content being viewed by others
Abbreviations
- NL:
-
Nail lacquers
- LCZ:
-
Luliconazole
- LCZ-NL:
-
Luliconazole NL
- AFM:
-
Atomic force microscope
- SEM:
-
Scanning electron microscope
- XRD:
-
X-ray diffraction
- DSC:
-
Differential scanning calorimetry
- T. rubrum :
-
Trichophyton rubrum
- T. mentagrophytes :
-
Trichophyton mentagrophytes
- USFDA:
-
United States Food and Drug Administration
- HPC-EF:
-
Hydroxypropyl cellulose EF
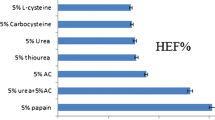
- HEF:
-
Hydration enhancement factor
References
Kataria P, Sharma G, Thakur K, Bansal V, Dogra S, Katare OP. Emergence of nail lacquers as potential transungual delivery system in the management of onchomycosis. Expert Opin Drug Deliv. 2016;13:937–52. https://doi.org/10.1080/17425247.2016.1174691.
Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835–51. https://doi.org/10.1016/j.jaad.2018.03.062.
Akhtar N, Sharma H, Pathak K. Onychomycosis: potential of nail lacquers in transungual delivery of antifungals. Scientifica (Cairo). 2016;2016:1387936. https://doi.org/10.1155/2016/1387936.
Ghannoum MA, Hajjeh RA, Scher R, Konnikov N, Gupta AK, Summerbell R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641–8. https://doi.org/10.1067/mjd.2000.107754.
Beuscher TL, Kelechi TJ. Onychomycosis: diagnosis, treatment, and prevention. J Wound Ostomy Continence Nurs. 2019;46:333–5. https://doi.org/10.1097/won.0000000000000556.
Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415–29. https://doi.org/10.1128/cmr.11.3.415.
Kaul S, Yadav S, Dogra S. Treatment of dermatophytosis in elderly, children, and pregnant women. Indian Dermatol Online J. 2017;8:310–8. https://doi.org/10.4103/idoj.IDOJ_169_17.
Eba M, Njunda AL, Mouliom RN, Kwenti ET, Fuh AN, Nchanji GT, et al. Onychomycosis in diabetic patients in Fako Division of Cameroon: prevalence, causative agents, associated factors and antifungal sensitivity patterns. BMC Res Notes. 2016;9:494. https://doi.org/10.1186/s13104-016-2302-1.
Gupta AK, Stec N, Summerbell RC, Shear NH, Piguet V, Tosti A, et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34:1972–90. https://doi.org/10.1111/jdv.16394.
Tolstrup J, Jemec GB, Hare RK, Arendrup MC, Saunte DM. Diagnosing and treating of onychomycosis. Ugeskr Laeger. 2018;180:1–5.
Negi S, Chaudhuri A, Kumar DN, Dehari D, Singh S, Agrawal AK. Nanotherapeutics in autophagy: a paradigm shift in cancer treatment. Drug Deliv Transl Res. 2022. https://doi.org/10.1007/s13346-022-01125-6.
Wiederhold NP, Fothergill AW, McCarthy DI, Tavakkol A. Luliconazole demonstrates potent in vitro activity against dermatophytes recovered from patients with onychomycosis. Antimicrob Agents Chemother. 2014;58:3553–5. https://doi.org/10.1128/aac.02706-13.
Vaezi A, Fakhim H, Arastehfar A, Shokohi T, Hedayati MT, Khodavaisy S, et al. In vitro antifungal activity of amphotericin B and 11 comparators against Aspergillus terreus species complex. Mycoses. 2018;61:134–42. https://doi.org/10.1111/myc.12716.
Anjum MM, Patel KK, Dehari D, Pandey N, Tilak R, Agrawal AK, et al. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: chitosan and DNase coating improves antimicrobial activity. Drug Deliv Transl Res. 2021;11:305–17. https://doi.org/10.1007/s13346-020-00795-4.
Dhamoon RK, Goyal RK, Popli H, Gupta M. Luliconazole-loaded thermosensitive hydrogel as aqueous based nail lacquer for the treatment of onychomycosis. Drug Deliv Lett. 2019;9:321–9.
Elsayed MM. Development of topical therapeutics for management of onychomycosis and other nail disorders: a pharmaceutical perspective. J Control Release. 2015;199:132–44. https://doi.org/10.1016/j.jconrel.2014.11.017.
Gupta AK, Foley KA, Versteeg SG. New antifungal agents and new formulations against dermatophytes. Mycopathologia. 2017;182:127–41. https://doi.org/10.1007/s11046-016-0045-0.
Das S, Lee SH, Chia VD, Chow PS, Macbeath C, Liu Y, et al. Development of microemulsion based topical ivermectin formulations: pre-formulation and formulation studies. Colloids Surf B Biointerfaces. 2020;189: 110823. https://doi.org/10.1016/j.colsurfb.2020.110823.
Kumar DN, Chaudhuri A, Aqil F, Dehari D, Munagala R, Singh S, et al. Exosomes as emerging drug delivery and diagnostic modality for breast cancer: recent advances in isolation and application. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14061435
Nedyalkova MA, Madurga S, Tobiszewski M, Simeonov V. Calculating the partition coefficients of organic solvents in octanol/water and octanol/air. J Chem Inf Model. 2019;59:2257–63. https://doi.org/10.1021/acs.jcim.9b00212.
Harde H, Agrawal AK, Jain S. Tetanus toxoid-loaded layer-by-layer nanoassemblies for efficient systemic, mucosal, and cellular immunostimulatory response following oral administration. Drug Deliv Transl Res. 2015;5:498–510. https://doi.org/10.1007/s13346-015-0247-x.
Jain S, Sharma JM, Agrawal AK, Mahajan RR. Surface stabilized efavirenz nanoparticles for oral bioavailability enhancement. J Biomed Nanotechnol. 2013;9(11):1862–74. https://doi.org/10.1166/jbn.2013.1683.
Gade S, Patel KK, Gupta C, Anjum MM, Deepika D, Agrawal AK, et al. An Ex Vivo evaluation of moxifloxacin nanostructured lipid carrier enriched in situ gel for transcorneal permeation on goat cornea. J Pharm Sci. 2019;108:2905–16. https://doi.org/10.1016/j.xphs.2019.04.005.
Patel KK, Gade S, Anjum M, Singh SK, Maiti P, Agrawal AK, et al. Effect of penetration enhancers and amorphization on transdermal permeation flux of raloxifene-encapsulated solid lipid nanoparticles: an ex vivo study on human skin. Appl Nanosci. 2019;9:1383–94.
Khengar RH, Jones SA, Turner RB, Forbes B, Brown MB. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm Res. 2007;24:2207–12. https://doi.org/10.1007/s11095-007-9368-3.
Chouhan P, Saini TR. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm. 2012;436:179–82. https://doi.org/10.1016/j.ijpharm.2012.06.020.
Carvajal-Vidal P, González-Pizarro R, Araya C, Espina M, Halbaut L, Gómez de Aranda I, et al. Nanostructured lipid carriers loaded with halobetasol propionate for topical treatment of inflammation: development, characterization, biopharmaceutical behavior and therapeutic efficacy of gel dosage forms. Int J Pharm. 2020;585:119480.
Aggarwal R, Targhotra M, Sahoo P, Chauhan MK. Efinaconazole nail lacquer for the transungual drug delivery: Formulation, optimization, characterization and in vitro evaluation. Journal of Drug Delivery Science Technology. 2020;60: 101998. https://doi.org/10.1016/j.jddst.2020.101998.
Thatai P, Sapra B. Transungual Gel of Terbinafine hydrochloride for the management of onychomycosis: formulation, optimization, and evaluation. AAPS PharmSciTech. 2017;18:2316–28. https://doi.org/10.1208/s12249-017-0711-7.
Patel MM, Vora ZM. Formulation development and optimization of transungual drug delivery system of terbinafine hydrochloride for the treatment of onychomycosis. Drug Deliv Transl Res. 2016;6:263–75. https://doi.org/10.1007/s13346-016-0287-x.
Souza AMS, Ribeiro RCA, Pinheiro G, Pinheiro FI, Oliveira WN, Souza L, et al. Polishing the therapy of onychomycosis induced by candida spp.: amphotericin B-Loaded Nail Lacquer. Pharmaceutics. 2021;13. https://doi.org/10.3390/pharmaceutics13060784
Rahman A, Aqil M, Ahad A, Imam SS, Qadir A, Ali A. Application of central composite design for the optimization of itraconazole loaded nail lacquer formulation. 3 Biotech. 2021;11:324.
Joshi M, Sharma V, Pathak K. Matrix based system of isotretinoin as nail lacquer to enhance transungal delivery across human nail plate. Int J Pharm. 2015;478:268–77. https://doi.org/10.1016/j.ijpharm.2014.11.050.
Baghel S, Nair VS, Pirani A, Sravani AB, Bhemisetty B, Ananthamurthy K, et al. Luliconazole-loaded nanostructured lipid carriers for topical treatment of superficial Tinea infections. Dermatol Ther. 2020;33: e13959. https://doi.org/10.1111/dth.13959.
Gregorí Valdes BS, Serro AP, Gordo PM, Silva A, Gonçalves L, Salgado A, et al. New polyurethane nail lacquers for the delivery of terbinafine: formulation and antifungal activity evaluation. J Pharm Sci. 2017;106:1570–7. https://doi.org/10.1016/j.xphs.2017.02.017.
Agrawal P, Singh RP, Sonali Kumari L, Sharma G, Koch B, et al. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater Sci Eng C Mater Biol Appl. 2017;74:167–76. https://doi.org/10.1016/j.msec.2017.02.008.
Patel KK, Surekha DB, Tripathi M, Anjum MM, Muthu MS, Tilak R, et al. Antibiofilm Potential of silver sulfadiazine-loaded nanoparticle formulations: a study on the effect of dnase-i on microbial biofilm and wound healing activity. Mol Pharm. 2019;16:3916–25. https://doi.org/10.1021/acs.molpharmaceut.9b00527.
Repka MA, O’Haver J, See CH, Gutta K, Munjal M. Nail morphology studies as assessments for onychomycosis treatment modalities. Int J Pharm. 2002;245:25–36. https://doi.org/10.1016/s0378-5173(02)00321-6.
Pawde DM, Viswanadh MK, Mehata AK, Sonkar R, Narendra Poddar S, et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm J. 2020;28:1616–25.
Shaikh MS, Kale MA, Shaikh MM, Mahaparale PR. Formulation, characterization and antimicrobial studies of lyophilized luliconazole nanosuspension for enhancing solubility using modified polymer. Int. J. Polym. Mater. Polym. Biomater. 2021;1–15. https://doi.org/10.1080/00914037.2021.1879077
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine. 2018;14:1629–41. https://doi.org/10.1016/j.nano.2018.04.009.
Kumar M, Shanthi N, Mahato AK, Soni S, Rajnikanth PS. Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon. 2019;5: e01688. https://doi.org/10.1016/j.heliyon.2019.e01688.
Mahmood A, Rapalli VK, Waghule T, Gorantla S, Singhvi G. Luliconazole loaded lyotropic liquid crystalline nanoparticles for topical delivery: QbD driven optimization, in-vitro characterization and dermatokinetic assessment. Chem Phys Lipids. 2021;234: 105028. https://doi.org/10.1016/j.chemphyslip.2020.105028.
Patel KK, Tripathi M, Pandey N, Agrawal AK, Gade S, Anjum MM, et al. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int J Pharm. 2019;563:30–42.
Koga H, Nanjoh Y, Makimura K, Tsuboi R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med Mycol. 2009;47:640–7. https://doi.org/10.1080/13693780802541518.
Shimamura T, Hasegawa N, Kubota N. Antifungal activity of luliconazole nail solution on in vitro and in vivo onychomycosis model. Med Mycol J. 2016;57:J13–8. https://doi.org/10.3314/mmj.57.J13.
Drug-Bank. Luliconazole. DrugBank Accession Number: DB08933. 2021. https://go.drugbank.com/drugs/DB08933. Accessed 22 July 2021.
Pragya V, Shikha A. Luliconazole emulgel: characterization, preparation, and evaluation. 2021;10:752–63. https://doi.org/10.20959/wjpr20217-20733
Garg AK, Maddiboyina B, Alqarni MHS, Alam A, Aldawsari HM, Rawat P, et al. Solubility enhancement, formulation development and antifungal activity of luliconazole niosomal gel-based system. J Biomater Sci Polym Ed. 2021;32:1009–23. https://doi.org/10.1080/09205063.2021.1892471.
Thatai P, Sapra B. Terbinafine hydrochloride nail lacquer for the management of onychomycosis: formulation, characterization and in vitro evaluation. Ther Deliv. 2018;9:99–119. https://doi.org/10.4155/tde-2017-0069.
Hui X, Shainhouse Z, Tanojo H, Anigbogu A, Markus GE, Maibach HI, et al. Enhanced human nail drug delivery: nail inner drug content assayed by new unique method. J Pharm Sci. 2002;91:189–95. https://doi.org/10.1002/jps.10003.
Cutrin-Gomez E, Anguiano-Igea S, Delgado-Charro MB, Gomez-Amoza JL, Otero-Espinar FJ. Effect of penetration enhancers on drug nail permeability from cyclodextrin/poloxamer-soluble polypseudorotaxane-based nail lacquers. Pharm. 2018;10. https://doi.org/10.3390/pharmaceutics10040273
Miyata Y, Masuda T. Luliconazole as anti-acanthamoeba agent and method for producing the same. WO2017094204A1. Google Patents. 2020. https://patents.google.com/patent/WO2017094204A1/en. Accessed 22 July 2021.
Mehata AK, Dehari D, Ayyannan SR, Muthu MS. X-ray powder diffraction spectroscopy as a robust tool in early predicting bioavailability of pharmaceutical formulation containing polymorphic drug substance. Drug Deliv Lett. 2020;10:250–4. https://doi.org/10.2174/2210303110999200519074306.
Acknowledgements
The authors are grateful to the Department of Pharmaceutical Engineering and Technology, Indian Institute of Technology (BHU), Varanasi–221005, India, for providing the infrastructure for successful execution of the current project.
Author information
Authors and Affiliations
Contributions
Deepa Dehari: conceptualization, experimentation literature survey, original draft writing. Abhishesh Kumar Mehata: compilation of data and editing. Vishnu Priya: review and editing. Dharmnath Parbat: literature survey and editing. Deepak Kumar: helped in antimicrobial studies. Anand Kumar Srivastava: Project administration. Ashish Kumar Agrawal: Conceptualization, project administration, overall modification, and correction.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dehari, D., Mehata, A.K., Priya, V. et al. Luliconazole Nail Lacquer for the Treatment of Onychomycosis: Formulation, Characterization and In Vitro and Ex Vivo Evaluation. AAPS PharmSciTech 23, 175 (2022). https://doi.org/10.1208/s12249-022-02324-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02324-7