Abstract
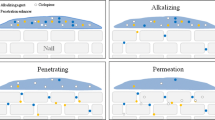
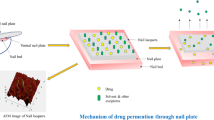
The aims of our investigation were to develop and optimize ciclopirox (CPX) nail lacquer using nonbiodegradable Eudragit RLPO (E-RLPO) as a film former and to assess its penetration efficiency across the human nail plate. Preliminary trials such as hydration enhancement factor (HEF), a retained drug in the nail plate, and SEM were studied to select the optimized permeation enhancer to be incorporated in the optimized lacquer formulation. A 33 full factorial design was built up to study the effect of three different factors, concentration of E-RLPO (10, 20, and 30%), Tween 80 (0.25, 0.5, and 1%), and triacetin (0, 10, and 30% of polymer weight). The studied responses were the drying time, water resistance, viscosity, and drug release up to 4 h. An ex vivo permeation study for the optimized formulations was carried out. The preliminary study aided the selection of 5% papain (endopeptidase enzyme) as a penetration enhancer; it showed the highest HEF of 15.27%, the highest amount of drug retained in the nail plate (886.2 μg/g). An ex vivo permeation study guided the selection of F4B (flux value of 3.79 μg/cm2/h) as optimized formulation. The optimized lacquer formula showed threefold increases in the permeation than the marketed CPX lacquer (Batrafen®). Confocal laser scanning microscopy revealed the higher intensity of the Rhodamine B dye across the nail plate in the case of the formula containing papain than the marketed formula without papain. Conclusively, an efficient and stable nail lacquer was developed for potential transungual delivery of CPX to target the drug to the nail bed and ensure efficiency against onychomycosis.









Similar content being viewed by others
References
Murdan S. Enhancing the nail permeability of topically applied drugs. Expert Opin Drug Deliv. 2008;5(11):267–1282.
Tanriverdi ST, Ozer O. Novel topical formulations of terbinafine-HCl for the treatment of onychomycosis. Eur J Pharm Sci. 2013;48(4–5):628–36.
Indr Š, Vitalis B. Effect of film-forming polymers on release of naftifine hydrochloride from nail lacquers. Int J Polym Sci. 2017;2017:1–7.
Naumann S, Meyer JP, Kiesow A, Mrestani Y, Wohlrab J, Neubert RHH, et al. Controlled nail delivery of a novel lipophilic antifungal agent using various modern drug carrier systems as well as in vitro and ex vivo model systems. J Control Release. 2014;180(1):60–70.
Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20(10):31–46.
Westerberg DP, Voyack MJ. Onychomycosis: current trends in diagnosis and management. Am Fam Physician (Review). 2013;88(11):762–70.
Jump UP, Chi CC, Wang SH, Chou MC. The causative pathogens of onychomycosis in southern Taiwan. Mycoses. 2005;48(6):413–20.
Elsayed MAA. Development of topical therapeutics for management of onychomycosis and other nail disorders: a pharmaceutical perspective. J Control Release. 2015;199:132–44.
Vejnovic I, Simmler L, Betz G. Investigation of different formulations for drug delivery through the nail plate. Int J Pharm. 2010;386(1–2):185–94.
Tietz HJ, Hay R, Querner S, Delcker A, Kurka P, Merk HF. Efficacy of 4weeks topical bifonazole treatment for onychomycosis after nail ablation with 40% urea: a double-blind, randomized, placebo-controlled multicenter study. Mycoses. 2013;56(4):414–21.
Rajendra VB, Baro A, Kumari A, Dhamecha DL, Lahoti SR, Shelke SD. Transungual drug delivery: an overview. J Appl Pharm Sci. 2012;2(1):203–9.
Frederiksen K, Guy RH, Petersson K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin Drug Deliv. 2016;13(3):349–60.
Saner MV, Kulkarni AD, Pardeshi CV. Insights into drug delivery across the nail plate barrier. J DrugTargtet. 2014;22(9):769–89.
Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone MF, Mailland F. In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev Ind Pharm. 2005;31(1):11–7.
Lunter DJ, Daniels R. New film forming emulsions containing Eudragit NE and/or RS 30D for sustained dermal delivery of nonivamide. Eur J Pharm Biopharm. 2012;82(2):291–8.
Felton LA. Mechanisms of polymeric film formation. Int J Pharm. 2013;457(2):423–7.
Hafeez F, Hui X, Chiang A, Hornby S, Maibach H. Transungual delivery of ketoconazole using novel lacquer formulation. Int J Pharm. 2013;456(2):357–61.
Monti D, Saccomani L, Chetoni P, Burgalassi S, Senesi S, Ghelardi E, et al. Hydrosoluble medicated nail lacquers: in vitro drug permeation and corresponding antimycoticactivity. Br J Dermatol. 2010;162(2):311–7.
Shireesh KR, Chandra SB, Vishnu P, Prasad MV. Ungual drug delivery systems of ketoconazole nail lacquer. Int J Appl Pharm. 2010;2:17–9.
Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev 2007, Issue 3. Art. No.: CD001434. doi: https://doi.org/10.1002/14651858.CD001434.pub2.
Palliyil B, Lebo DB, Patel P. A preformulation strategy for the selection of penetration enhancers for a transungual formulation. AAPS PharmSciTech. 2013;14(2):682–91.
Monika J, Vijay S, Kamla P. Matrix based system of isotretinoin as nail lacquer to enhance transungual delivery across human nail plate. Int J Pharm. 2014;478(1):268–77.
Noha IE, Rehab NS, Ghada A. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: in vitro characterization and ex vivo evaluation. AAPS PharmSciTech. 2016;18(2):551–61.
Gardner HA. Physical examination of paints, varnishes, lacquers and colors. Bethesda: Gardner Laboratory, Inc.; 1956.
Noha MZ, Gehanne AA, Nahed DM, Seham SA. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesivein situ gel with modulated rheological and mucociliary transport properties. Eur J Pharm Biopharm. 2007;32:296–307.
Biji P, David BL. A novel transungual formulation (nail patch) for delivery of ciclopirox olamine into the nail and the nail folds. Int J pharm Med Biol Sci. 2014;3(2):152–64.
Chouhan P, Saini TR. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm. 2012;436:179–82.
Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26.
VanHoogdalem EJ, Van den Hoven WE, Terpstra IJ, ZijtveldJ V, Verschoor JSC, Visser JN. Nail penetration of the antifungal agent oxiconazole after repeated topical application in healthy volunteers, and the effect of acetylcysteine. Eur J Pharm Sci. 1997;5:119–27.
Kobayashi Y, Miyamoto M, Sugibayashi K, Morimoto Y. Enhancing effect of N- acetyl-L- cysteine or 2- mercaptoethanol on the in vitro permeation of 5- flurouracil or tolnaftate through the human nail plate. Chem Pharm Bull. 1998;46:1797–802.
Bhagat S, Agarwal M, Roy V. Serratiopeptidase: a systematic review of the existing evidence. In J Surg. 2010;11:209–17.
Elkeeb R, Alikhan A, Elkeeb L, Hui X, Maibach H. Transungual drug delivery current status. Int J Pharm. 2010;384:1–8.
Shivakumar HN, Juluri A, Desai BG, Murthy SN. Ungual and transungual drug delivery. Drug Dev Ind Pharm. 2012;38(8):901–11.
Nida A, Hemlata S, Kamla P. Onychomycosis: potential of nail lacquers in transungual delivery of antifungals. Scientifica. 2016;2016:1–12.
Melissa GAV, Mariana AS, Lucielen OS. Natural-based plasticizers and biopolymer films: a review. Eur Polym J. 2011;47(3):254–63.
Rajan RS, Jasmina K, Arunabha N. Studies on the effect of plasticizer on in vitro release and ex vivo permeation from Eudragit E 100 based chlorpheniramine maleate matrix type transdermal delivery system. J Excipients Food Chem. 2010;1(2):1–12.
Schlossman M. Techniques for evaluation of nail enamel. J Soc Cosmet Chem. 1981;32:43–52.
Sharma PP. Cosmetics—formulation, manufacturing and quality control. 2008; 4th edition Vandana publications (India) Pvt. Ltd., New Delhi.
Sinko PJ. Physical pharmacy and pharmaceutical sciences. 5th ed. New Delhi: Wolters Kluwer (India) Pvt. Ltd; 2006.
Gutierrez-Rocca JC, McGinity JW. Influence of aging on the physical mechanical properties of acrylic resin films cast from aqueous dispersion and organic solution. Drug Dev Ind Pharm. 1993;19:315–32.
ASTM (2016) D3924-16, standard specification for standard environment for conditioning and testing paint varnish lacquer, and related materials, Varnish, ASTM International, West Conshohocken, PA, www.astm.org.
Owen DH, Peters JJ, Katz DF. Rheological properties of contraceptive gels. Contraception. 2000;62:321–6.
Chang JY, Oh Y, Choi HG, Kim YB, Kim CK. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. 2002;241:155–63.
Balsam MS, Edward S. Nail lacquer and remover. In: Balsam MS, Sagarine E, Strianse J, Gershon SD, Rieger MM, editors. Cosmetic science and technology. 2nd edition. Wiley-Interscience, a division of John Wiley and Sons Inc, New York; (1972) Volume 2, chapter (29): p. 521–541.
Hung SF, Hsiech C, Chen CY, Wang YC, Sheu MT. Characterizations of plasticized polymeric film coatings for preparing multiple-unit floating drug delivery systems (muFDDSs) with controlled-release characteristics. PLoS One. 2014;9(6):e100321.
Frederiksen K, Guy RH, Petersson K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur J Pharm Biopharm. 2015;91:9–15.
Subissi A, Monti D, Togni G, Mailland F. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70(16):2133–52.
Schroeder IZ, Franke P, Schaefer UF, Lehr CM. Delivery of ethinyl estradiol from film forming polymeric solutions across human epidermis in vitro and in vivo in pigs. J Control Release. 2007;118(2):196–203.
Padula C, Nicoli S, Colombo P, Santi P. Single-layer transdermal film containing lidocaine: modulation of drug release. Eur J Pharm Biopharm. 2007;66(3):422–8.
Murdan S, Kerai L, Hossin B. To what extent do in vitro tests correctly predict the in vivo residence of nail lacquers on the nail plate? J Drug Deliv Sci Technol. 2015;25:23–8.
Lecomte F, Siepmann J, Walther M, MacRae R, Bodmeier R. Polymer blends used for the coating of multiparticulates: comparison of aqueous and organic coating techniques. Pharm Res. 2004;21(5):882–90.
Quaddir MA, Reza MS, Haider SS. Effect of polyethylene glycols on release of diclofenac sodium from directly compressed carnauba wax matrix tablets. J Bang Acad Sci. 2002;26(1):1–8.
Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends used for the aqueous coating of solid dosage forms: importance of the type of plasticizer. J Control Release. 2004;99(1):1–13.
Ye Z, Rombout P, Remon JP, Vervaet C, Van den Mooter G. Correlation between the permeability of metoprolol tartrate through plasticized isolated ethylcellulose/hydroxypropyl methylcellulose films and drug release from reservoir pellets. Eur J Pharm Biopharm. 2007;67:485–90.
Bodmeier R, Paeratakul O. Leaching of water-soluble plasticizers from polymeric films prepared from aqueous colloidal polymer dispersions. Drug Dev Ind Pharm. 1992;18(17):1865–82.
Hyppölä R, Husson I, Sundholm F. Evaluation of physical properties of plasticized ethyl cellulose films cast from ethanol solution part I. Int J Pharm. 1996;133:161–70.
Rowe RC. The effect of the molecular weight of ethyl cellulose on the drug release properties of mixed films of ethyl cellulose and hydroxypropyl methylcellulose. Int J Pharm. 1986;29:37–41.
22Seattone MF, Perini G, Rijli P, Rodriguez L, Cini M. Effect of different polymer-plasticizer combinations on in vitro release of theophylline from coated pellets. Int J Pharm. 1995;126:83–8.
Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228(2):161–70.
Walters KA, Brain KR, Green DM, James VG, Atkinson AC, Sands RH. Comparison of the transdermal delivery of estradiol from two gel formulations. Maturitas. 1998;29:189–95.
Acknowledgments
The authors are thankful to Röhm GMbH Chemische Fabrik, Germany, for providing the gift sample of E-RLPO. The authors would like also to acknowledge the National Organization for Drug Control and Research for providing all necessary facilities during project work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khattab, A., Shalaby, S. Optimized Ciclopirox-Based Eudragit RLPO Nail Lacquer: Effect of Endopeptidase Enzyme as Permeation Enhancer on Transungual Drug Delivery and Efficiency Against Onychomycosis. AAPS PharmSciTech 19, 1048–1060 (2018). https://doi.org/10.1208/s12249-017-0917-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0917-8