Abstract
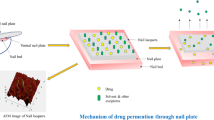
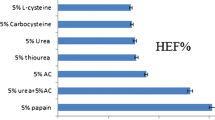
Onychomycosis is a common fungal infection of the nails that mostly affects the elderly and athletes. Antifungal drug-like itraconazole is one of the therapeutic agents of choice for the topical treatment of onychomycosis. The current work aimed for the preparation and optimization of itraconazole-loaded nail lacquer formulation. Central composite design was employed; independent variables were polymer concentration (X1) and thioglycolic acid (TGA) concentration (X2). While the dependent variables were cumulative amount of drug permeated per unit area (CADP/A, Y1), drying time (Y2) and nonvolatile content (Y3). The optimized formulation was characterized for various parameters including ex-vivo permeation study, confocal laser scanning microscopy (CLSM) and antifungal study. The optimized nail lacquer formulation (F7) exhibited CADP/A of 198.23 µg/cm2, drying time of 185 s and nonvolatile content of 97.23%. The scanning electron microscopy of goat hoof treated with optimized nail lacquer formulation demonstrated loosening of the structure and marked increase in surface roughness. The CLSM micrograph of goat hoof treated with optimized nail lacquer formulation demonstrated that the probe dye was eventually distributed and penetrated through the hoof. Bio-adhesiveness analysis showed that the prepared nail lacquer film has ample adhesiveness to be maintained on the nail plate surface for a sufficient time. In antifungal study, the optimized nail lacquer, and marketed gel exhibited a zone inhibition of 21 mm, and 10 mm against Candida albicans, respectively. The stability study showed that the optimized nail lacquer is stable at storage condition. The prepared nail lacquers have been shown to serve as a useful dosage form for the delivery of itraconazole across nail plate for controlling the problems associated with onychomycosis.








Similar content being viewed by others
Availability of data and material
The authors confirm that the data findings of this study are available within the article.
References
Akhtar N, Sharma H, Pathak K (2016) Onychomycosis: potential of nail lacquers in transungual delivery of antifungals. Scientifica (cairo) 2016:1387936. https://doi.org/10.1155/2016/1387936
Ameeduzzafar QM, Alruwaili NK, Bukhari SNA, Alharbi KS, Imam SS, Afzal M, Alsuwayt B, Mujtaba A, Ali A (2021) BBD-based development of itraconazole loaded nanostructured lipid carrier for topical delivery: in vitro evaluation and antimicrobial assessment. J Pharm Innov 16:85–98
Bseiso EA, Nasr M, Sammour OA, Abd El Gawad NA (2016) Novel nail penetration enhancer containing vesicles “nPEVs” for treatment of onychomycosis. Drug Deliv 23:2813–2819
Chouhan P, Saini TR (2012) Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm 436:179–182
Christenson JK, Peterson GM, Naunton M, Bushell M, Kosari S, Baby KE, Thomas J (2018) Challenges and opportunities in the management of onychomycosis. J Fungi (basel). https://doi.org/10.3390/jof4030087
Cutrin-Gomez E, Anguiano-Igea S, Delgado-Charro MB, Gomez-Amoza JL, Otero-Espinar FJ (2018) Effect of penetration enhancers on drug nail permeability from cyclodextrin/poloxamer-soluble polypseudorotaxane-based nail lacquers. Pharmaceutics. https://doi.org/10.3390/pharmaceutics10040273
Del Rosso JQ (2014) The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol 7:10–18
Dhamoon RK, Popli H, Gupta M (2019) Novel drug delivery strategies for the treatment of onychomycosis. Pharm Nanotechnol 7:24–38
Elewski BE (1998) Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev 11:415–429
Faergemann J, Baran R (2003) Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol 149(Suppl 65):1–4
Feng X, Xiong X, Ran Y (2017) Efficacy and tolerability of amorolfine 5% nail lacquer in combination with systemic antifungal agents for onychomycosis: A meta-analysis and systematic review. Dermatol Ther. https://doi.org/10.1111/dth.12457
Ghannoum M, Isham N (2014) Fungal nail infections (onychomycosis): a never-ending story? PLoS Pathog 10:e1004105. https://doi.org/10.1371/journal.ppat.1004105
Ghannoum M, Long L, Kunze G, Sarkany M, Osman-Ponchet H (2019) A pilot, layerwise, ex vivo evaluation of the antifungal efficacy of amorolfine 5% nail lacquer vs other topical antifungal nail formulations in healthy toenails. Mycoses 62:494–501
Gupta AK, Stec N (2019) Recent advances in therapies for onychomycosis and its management [version 1; peer review: 2 approved]. F1000Res 8(F1000 Faculty Rev):968. https://doi.org/10.12688/f1000research.18646.1
Hasan N, Singh M, Sulaiman S, Nandy S, Dudeja M, Ali A, Iqbal Z (2018) Design, development and optimization of a transungual duple nail lacquer for onychomycosis therapy. J Eur Acad Dermatol Venereol 32:e250–e251
Hassan N, Singh M, Sulaiman S, Jain P, Sharma K, Nandy S, Dudeja M, Ali A, Iqbal Z (2019) Molecular docking-guided ungual drug-delivery design for amelioration of onychomycosis. ACS Omega 4:9583–9592
Hoda Q, Aqil M, Ahad A, Imam SS, Praveen A, Qadir A, Iqbal Z (2021) Optimization of valencene containing lipid vesicles for boosting the transungual delivery of itraconazole. Biotech 11:137. https://doi.org/10.1007/s13205-020-02497-7
Joshi M, Sharma V, Pathak K (2015) Matrix based system of isotretinoin as nail lacquer to enhance transungal delivery across human nail plate. Int J Pharm 478:268–277
Kushwaha A, Jacob M, Shiva Kumar HN, Hiremath S, Aradhya S, Repka MA, Murthy SN (2015) Trans-ungual delivery of itraconazole hydrochloride by iontophoresis. Drug Dev Ind Pharm 41:1089–1094
Lin H, Xie Q, Huang X, Ban J, Wang B, Wei X, Chen Y, Lu Z (2018) Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int J Nanomed 13:831–842
McAuley WJ, Jones SA, Traynor MJ, Guesne S, Murdan S, Brown MB (2016) An investigation of how fungal infection influences drug penetration through onychomycosis patient’s nail plates. Eur J Pharm Biopharm 102:178–184
Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone MF, Mailland F (2005) In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev Ind Pharm 31:11–17
Monti D, Saccomani L, Chetoni P, Burgalassi S, Senesi S, Ghelardi E, Mailland F (2010) Hydrosoluble medicated nail lacquers: in vitro drug permeation and corresponding antimycotic activity. Br J Dermatol 162:311–317
Murdan S (2002) Drug delivery to the nail following topical application. Int J Pharm 236:1–26
Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, Friden PM, Murthy SN (2009) Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci 98:4130–4140
Nesseem DI (2001) Formulation and evaluation of itraconazole via liquid crystal for topical delivery system. J Pharm Biomed Anal 26:387–399
Piraccini BM, Iorizzo M, Lencastre A, Nenoff P, Rigopoulos D (2020) Ciclopirox hydroxypropyl chitosan (HPCH) nail lacquer: a review of its use in onychomycosis. Dermatol Ther (heidelb) 10:917–929
Saner MV, Kulkarni AD, Pardeshi CV (2014) Insights into drug delivery across the nail plate barrier. J Drug Target 22:769–789
Shah VH, Jobanputra A (2018) Enhanced ungual permeation of terbinafine HCl delivered through liposome-loaded nail lacquer formulation optimized by QbD approach. AAPS PharmSciTech 19:213–224
Shirwaikar AA, Thomas T, Shirwaikar A, Lobo R, Prabhu KS (2008) Treatment of onychomycosis: an update. Indian J Pharm Sci 70:710–714
Shivakumar HN, Vaka SR, Madhav NV, Chandra H, Murthy SN (2010) Bilayered nail lacquer of terbinafine hydrochloride for treatment of onychomycosis. J Pharm Sci 99:4267–4276
Shivakumar HN, Juluri A, Desai BG, Murthy SN (2012) Ungual and transungual drug delivery. Drug Dev Ind Pharm 38:901–911
Tanriverdi ST, Hilmioglu Polat S, Yesim Metin D, Kandiloglu G, Ozer O (2016) Terbinafine hydrochloride loaded liposome film formulation for treatment of onychomycosis: in vitro and in vivo evaluation. J Liposome Res 26:163–173
Thapa RK, Choi JY, Go TG, Kang MH, Han SD, Jun JH, Son MW, Yong CS, Kim JO (2016) Development of ciclopirox nail lacquer with enhanced permeation and retention. Arch Pharm Res 39:953–959
Thatai P, Sapra B (2018) Terbinafine hydrochloride nail lacquer for the management of onychomycosis: formulation, characterization and in vitro evaluation. Ther Deliv 9:99–119
Vanden Bossche H, Marichal P, Le Jeune L, Coene MC, Gorrens J, Cools W (1993) Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother 37:2101–2105
Vipin KV, Sarath CC, Ann RA, Premaletha K, Kuriakose MR (2014) Formulation and evaluation of an antifungual nail lacquer for onychomycosis. Br Biomed Bull 2:242–248
Zeb A, Qureshi OS, Kim HS, Cha JH, Kim JK (2016) Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int J Nanomedicine 11:3813–3824
Funding
None.
Author information
Authors and Affiliations
Contributions
AR: Investigation, formulation, methodology. MA: Ideas, supervision, conceptualization. AA: Software, writing original draft preparation. SSI: Software, interpreting the data. AQ: Data collection. AALI: Ideas, design of methodology.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors report no declarations of interest.
Ethics approval and consent to participate
None.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Rahman, A., Aqil, M., Ahad, A. et al. Application of central composite design for the optimization of itraconazole loaded nail lacquer formulation. 3 Biotech 11, 324 (2021). https://doi.org/10.1007/s13205-021-02862-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02862-0