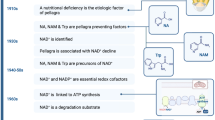
Abstract
The role of nicotinamide adenine dinucleotide (NAD+) in ageing has emerged as a critical factor in understanding links to a wide range of chronic diseases. Depletion of NAD+, a central redox cofactor and substrate of numerous metabolic enzymes, has been detected in many major age-related diseases. However, the mechanisms behind age-associated NAD+ decline remains poorly understood. Despite limited conclusive evidence, supplements aimed at increasing NAD+ levels are becoming increasingly popular. This review provides renewed insights regarding the clinical utility and benefits of NAD+ precursors, namely nicotinamide (NAM), nicotinic acid (NA), nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), in attenuating NAD+ decline and phenotypic characterization of age-related disorders, including metabolic, cardiovascular and neurodegenerative diseases. While it is anticipated that NAD+ precursors can play beneficial protective roles in several conditions, they vary in their ability to promote NAD+ anabolism with differing adverse effects. Careful evaluation of the role of NAD+, whether friend or foe in ageing, should be considered.


Similar content being viewed by others
References
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2015. (ST/ESA/SER.A/390). New York: United Nations; 2015.
National Institute on Aging. Global Aging [Internet]. United States: United States Government. 2022. https://www.nia.nih.gov/research/dbsr/global-aging#:~:text=Rapid%20declines%20in%20fertility%2C%20together%20with%20rising%20life,of%2065%20as%20under%20the%20age%20of%2015. Accessed 14 Jun 2022.
United Nations Department of Economic and Social Affairs. World Population Ageing 2019 [Internet]. United Nations. 2019. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Accessed 14 Jun 2022
Fang EF, Xie C, Schenkel JA, Wu C, Long Q, Cui H, et al. A research agenda for ageing in China in the 21st century (2nd edition): focusing on basic and translational research, long-term care, policy and social networks. Ageing Res Rev. 2020;64: 101174. https://doi.org/10.1016/j.arr.2020.101174.
Kc S, Wurzer M, Speringer M, Lutz W. Future population and human capital in heterogeneous India. Proc Natl Acad Sci USA. 2018;115(33):8328–33. https://doi.org/10.1073/pnas.1722359115.
Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:335. https://doi.org/10.3389/fpubh.2017.00335.
Warmoth K, Tarrant M, Abraham C, Lang IA. Older adults’ perceptions of ageing and their health and functioning: a systematic review of observational studies. Psychol Health Med. 2016;21(5):531–50. https://doi.org/10.1080/13548506.2015.
Rowe JW, Kahn RL. Successful aging. New York: Pantheon Books; 1998.
Fernández-Ballesteros R, García LF, Abarca D, Blanc L, Efklides A, Kornfeld R, et al. Lay concept of aging well: cross-cultural comparisons. J Am Geriatr Soc. 2008;56:950–2. https://doi.org/10.1111/j.1532-5415.2008.01654.x.
Schultz MB, Sinclair DA. Why NAD(+) declines during aging: it’s destroyed. Cell Metab. 2016;23(6):965–6. https://doi.org/10.1016/j.cmet.2016.05.022.
Cantó C, Menzies KJ, Auwerx J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53.
Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–71. https://doi.org/10.1016/j.tcb.2014.04.002.
Freeberg KA, Craighead DH, Martens CR, You Z, Chonchol M, Seals DR. Nicotinamide riboside supplementation for treating elevated systolic blood pressure and arterial stiffness in midlife and older adults. Front Cardiovasc Med. 2022;9: 881703. https://doi.org/10.3389/fcvm.2022.881703.
Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–41. https://doi.org/10.1038/s41580-020-00313-x/.
McReynolds MR, Chellappa K, Baur JA. Age-related NAD+ decline. Exp Gerontol. 2020;22(134): 110888. https://doi.org/10.1016/j.exger.2020.110888.
Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL, Bohr VA. Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol. 2016;17(5):308–21. https://doi.org/10.1038/nrm.2016.14.
Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157(4):882–96. https://doi.org/10.1016/j.cell.2014.03.026.
Strømland Ø, Diab J, Ferrario E, Sverkeli LJ, Ziegler M. The balance between NAD+ biosynthesis and consumption in ageing. Mech Ageing Dev. 2021;199: 111569. https://doi.org/10.1016/j.mad.2021.111569.
Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 2018;27(3):529–47. https://doi.org/10.1016/j.cmet.2018.02.011.
Harden A, Young WJ. The alcoholic ferment of yeast-juice part II–the coferment of yeast-juice. Proc R Soc Lond Ser B Contain Pap Biol Character. 1906;78:369–75.
Warburg O, Christian W. Pyridin, the hydrogen-transferring component of the fermentation enzymes (pyridine nucleotide). Biochem Zeitschr. 1936;287:291.
Shade C. The science behind NMN-A Stable, Reliable NAD+Activator and anti-aging molecule. Integr Med (Encinitas). 2020;19(1):12–4.
Castro-Portuguez R, Sutphin GL. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol. 2020;132: 110841. https://doi.org/10.1016/j.exger.2020.110841.
Lundt S, Ding S. NAD+ metabolism and diseases with motor dysfunction. Genes (Basel). 2021;12(11):1776. https://doi.org/10.3390/genes12111776.
Kennedy BE, Sharif T, Martell E, Dai C, Kim Y, Lee PW, et al. NAD+ salvage pathway in cancer metabolism and therapy. Pharmacol Res. 2016;114:274–83. https://doi.org/10.1016/j.phrs.2016.10.027.
Mahan DC, Shields RG. Essential and nonessential amino acid composition of pigs from birth to 145 kilograms of body weight, and comparison to other studies. J Anim Sci. 1998;76:513–21.
Hopkins F. The analyst and the medical man. Analyst. 1906;31:385b–404.
Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–7.
O’Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29(13):4200–9. https://doi.org/10.1523/JNEUROSCI.5032-08.2009.
Souza LC, Jesse CR, Antunes MS, Ruff JR, de Oliveira ED, Gomes NS, et al. Indoleamine-2,3-dioxygenase mediates neurobehavioral alterations induced by an intracerebroventricular injection of amyloid-β1-42 peptide in mice. Brain Behav Immun. 2016;56:363–77. https://doi.org/10.1016/j.bbi.2016.03.002.
Green AR, Sourkes TL, Young SN. Liver and brain tryptophan metabolism following hydrocortisone administration to rats and gerbils. Br J Pharmacol. 1975;53:287–92.
Nakamura T, Niimi S, Nawa K, Noda C, Ichihara A, Takagi Y, et al. Multihormonal regulation of transcription of the tryptophan 2,3-dioxygenase gene in primary cultures of adult rat hepatocytes with special reference to the presence of a transcriptional protein mediating the action of glucocorticoids. J Biol Chem. 1987;262:727–33.
Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37(8):684–7. https://doi.org/10.1016/j.clinbiochem.2004.02.007.
Pertovaara M, Raitala A, Lehtimäki T, Karhunen PJ, Oja SS, Jylhä M, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–9.
Pedersen ER, Svingen GF, Schartum-Hansen H, Ueland PM, Ebbing M, Nordrehaug JE, et al. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J. 2013;34:2689–96.
Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. https://doi.org/10.1111/j.1365-2990.2005.00655.x.
Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, et al. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human alzheimer’s disease brain. PLoS ONE. 2013;8(4): e59749. https://doi.org/10.1371/journal.pone.0059749.
Fertan E, Stover KRJ, Brant MG, Stafford PM, Kelly B, Diez-Cecilia E, et al. Effects of the novel IDO inhibitor DWG-1036 on the behavior of male and female 3xTg-AD mice. Front Pharmacol. 2019;10:1044. https://doi.org/10.3389/fphar.2019.01044.
Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry. 2016;6(1):54–65. https://doi.org/10.5498/wjp.v6.i1.54.
Dobrovolsky VN, Bowyer JF, Pabarcus MK, Heflich RH, Williams LD, Doerge DR, et al. Effect of arylformamidase (kynurenine formamidase) gene inactivation in mice on enzymatic activity, kynurenine pathway metabolites and phenotype. Biochim Biophys Acta. 2005;1724(1–2):163–72. https://doi.org/10.1016/j.bbagen.2005.03.010.
Hugill AJ, Stewart ME, Yon MA, Probert F, Cox IJ, Hough TA, et al. Loss of arylformamidase with reduced thymidine kinase expression leads to impaired glucose tolerance. Biol Open. 2015;4(11):1367–75. https://doi.org/10.1242/bio.013342.
Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences–an overview. Nutr Hosp. 2007;22(1):7–24.
Hvas AM, Juul S, Bech P, Nexø E. Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom. 2004;73(6):340–3.
McCarty MF. High-dose pyridoxine as an “anti-stress” strategy. Med Hypotheses. 2000;54(5):803–7. https://doi.org/10.1054/mehy.1999.0955.
Hansson O. Tryptophan load test and pyridoxine treatment in epileptic children. Acta Neurol Scand. 1967;43(S31):65. https://doi.org/10.1111/j.1600-0404.
Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1159–68. https://doi.org/10.1152/ajpregu.00767.2006.
Pierozan P, Zamoner A, Soska AK, Silvestrin RB, Loureiro SO, Heimfarth L, et al. Acute intrastriatal administration of quinolinic acid provokes hyperphosphorylation of cytoskeletal intermediate filament proteins in astrocytes and neurons of rats. Exp Neurol. 2010;224(1):188–96. https://doi.org/10.1016/j.expneurol.2010.03.009.
Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31(4):395–404. https://doi.org/10.1111/j.1365-2990.2005.00655.x.
Rahman A, Rao MS, Khan KM. Intraventricular infusion of quinolinic acid impairs spatial learning and memory in young rats: a novel mechanism of lead-induced neurotoxicity. J Neuroinflamm. 2018;15(1):263. https://doi.org/10.1186/s12974-018-1306-2.
Latif-Hernandez A, Shah D, Ahmed T, Lo AC, Callaerts-Vegh Z, Van der Linden A, Balschun D, D’Hooge R. Quinolinic acid injection in mouse medial prefrontal cortex affects reversal learning abilities, cortical connectivity and hippocampal synaptic plasticity. Sci Rep. 2016;6:36489. https://doi.org/10.1038/srep36489.
Roberts Buceta PM, Romanelli-Cedrez L, Babcock SJ, Xun H, VonPaige ML, Higley TW, et al. The kynurenine pathway is essential for rhodoquinone biosynthesis in Caenorhabditis elegans. J Biol Chem. 2019;294(28):11047–53. https://doi.org/10.1074/jbc.AC119.009475.
Mehmel M, Jovanović N, Spitz U. Nicotinamide riboside-the current state of research and therapeutic uses. Nutrients. 2020;12(6):1616. https://doi.org/10.3390/nu12061616.
Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754–63. https://doi.org/10.1074/jbc.M408388200.
Reiten OK, Wilvang MA, Mitchell SJ, Hu Z, Fang EF. Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech Ageing Dev. 2021;199: 111567. https://doi.org/10.1016/j.mad.2021.111567.
Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–82.
Ho C, van der Veer E, Akawi O, Pickering JG. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 2009;583:3081–5.
Imai S, The NAD. World: a new systemic regulatory network for metabolism and aging–Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74.
Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–26.
Hsu CP, Odewale I, Alcendor RR, Sadoshima J. Sirt1 protects the heart from aging and stress. Biol Chem. 2008;389:221–31.
Audrito V, Messana VG, Deaglio S. NAMPT and NAPRT: two metabolic enzymes with key roles in inflammation. Front Oncol. 2020;10:358. https://doi.org/10.3389/fonc.2020.00358.
Hara N, Yamada K, Shibata T, Osago H, Tsuchiya M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS ONE. 2011;6(8): e22781. https://doi.org/10.1371/journal.pone.0022781.
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–7.
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30.
Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6(5):363–75. https://doi.org/10.1016/j.cmet.2007.09.003.
Körner A, Garten A, Blüher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92(12):4783–91. https://doi.org/10.1210/jc.2007-1304.
Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15(3):26–33. https://doi.org/10.1111/dom.12171.
Choi YJ, Choi SE, Ha ES, Kang Y, Han SJ, Kim DJ, et al. Extracellular visfatin activates gluconeogenesis in HepG2 cells through the classical PKA/CREB-dependent pathway. Horm Metab Res. 2014;46(4):233–9. https://doi.org/10.1055/s-0034-1370907.
Kim SR, Bae SK, Choi KS, Park SY, Jun HO, Lee JY, et al. Visfatin promotes angiogenesis by activation of extracellular signal-regulated kinase 1/2. Biochem Biophys Res Commun. 2007;357(1):150–6. https://doi.org/10.1016/j.bbrc.2007.03.105.
Ma C, Pi C, Yang Y, Lin L, Shi Y, Li Y, et al. Nampt expression decreases age-related senescence in rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS ONE. 2017;12(1): e0170930. https://doi.org/10.1371/journal.pone.0170930.
Nakajima TE, Yamada Y, Hamano T, Furuta K, Gotoda T, Katai H, et al. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol. 2009;44(7):685–90. https://doi.org/10.1007/s00535-009-0063-5.
Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–80.
Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–95. https://doi.org/10.1002/art.21942.
Franco-Trepat E, Alonso-Perez A, Guillan-Fresco M, Jorge-Mora A, Gualillo O, Gomez-Reino JJ, et al. Visfatin as a therapeutic target for rheumatoid arthritis. Expert Opin Ther Targets. 2019;23:607–18. https://doi.org/10.1080/14728222.2019.1617274.
Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS ONE. 2008;3: e2267. https://doi.org/10.1371/journal.pone.0002267.
Neubauer K, Bednarz-Misa I, Walecka-Zacharska E, Wierzbicki J, Agrawal A, Gamian A, et al. Oversecretion and overexpression of nicotinamide phosphoribosyltransferase/Pre-B colony-enhancing factor/visfatin in inflammatory bowel disease reflects the disease activity, severity of inflammatory response and hypoxia. Int J Mol Sci. 2019;20:166.
Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–27. https://doi.org/10.1002/dmrr.1201.
Nielsen KN, Peics J, Ma T, Karavaeva I, Dall M, Chubanava S, et al. NAMPT-mediated NAD(+) biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol Metab. 2018;11:178–88. https://doi.org/10.1016/j.molmet.2018.02.014.
Sayers SR, Beavil RL, Fine NHF, Huang GC, Choudhary P, Pacholarz KJ, et al. Structure-functional changes in eNAMPT at high concentrations mediate mouse and human beta cell dysfunction in type 2 diabetes. Diabetologia. 2019;63:313–23. https://doi.org/10.1007/s00125-019-05029-y.
Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11(9):535–46. https://doi.org/10.1038/nrendo.2015.117.
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Retraction Science. 2007;318:565.
Dahl TB, Ynestad A, Skjelland M, Øie E, Dahl A, Michelsen A, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–80.
Song HK, Lee MH, Kim BK, Park YG, Ko GJ, Kang YS, et al. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1485–94.
Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–10.
Xing S, Hu Y, Huang X, Shen D, Chen C. Nicotinamide phosphoribosyltransferase-related signaling pathway in early Alzheimer’s disease mouse models. Mol Med Rep. 2019;20(6):5163–71.
Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269(20):14835–40.
Pissios P. Nicotinamide N-methyltransferase: more than a vitamin B3 clearance enzyme. Trends Endocrinol Metab. 2017;28(5):340–53. https://doi.org/10.1016/j.tem.2017.02.004.
Kang-Lee YA, McKee RW, Wright SM, Swendseid ME, Jenden DJ, Jope RS. Metabolic effects of nicotinamide administration in rats. J Nutr. 1983;113(2):215–21. https://doi.org/10.1093/jn/113.2.215.
Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9(11):693–700. https://doi.org/10.1038/nchembio.1352.
Parsons RB, Smith SW, Waring RH, Williams AC, Ramsden DB. High expression of nicotinamide N-methyltransferase in patients with idiopathic Parkinson’s disease. Neurosci Lett. 2003;342(1–2):13–6.
Liu KY, Mistry RJ, Aguirre CA, Fasouli ES, Thomas MG, Klamt F, et al. Nicotinamide N-methyltransferase increases complex I activity in SH-SY5Y cells via sirtuin 3. Biochem Biophys Res Commun. 2015;467(3):491–6. https://doi.org/10.1016/j.bbrc.2015.10.023.
Parsons RB, Aravindan S, Kadampeswaran A, Evans EA, Sandhu KK, Levy E, et al. The expression of nicotinamide N-methyltransferas increases ATP synthesis and protects SH-SY5Y neuroblastoma cells against the toxicity of complex I inhibitors. Biochem J. 2011;436(1):145–55. https://doi.org/10.1042/BJ20101685.
Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp. 2008;68(1):1–9.
Schmeisser K, Parker JA. Nicotinamide-N-methyltransferase controls behavior, neurodegeneration and lifespan by regulating neuronal autophagy. PLoS Genet. 2018;14(9): e1007561. https://doi.org/10.1371/journal.pgen.1007561.
Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495–502.
Sambeat A, Ratajczak J, Joffraud M, Sanchez-Garcia JL, Giner MP, Valsesia A, et al. Endogenous nicotinamide riboside metabolism protects against diet-induced liver damage. Nat Commun. 2019;10(1):4291. https://doi.org/10.1038/s41467-019-12262-x.
Yang Y, Mohammed FS, Zhang N, Sauve AA. Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J Biol Chem. 2019;294(23):9295–307. https://doi.org/10.1074/jbc.RA118.005772.
Megarity CF, Gill JR, Caraher MC, Stratford IJ, Nolan KA, Timson DJ. The two common polymorphic forms of human NRH-quinone oxidoreductase 2 (NQO2) have different biochemical properties. FEBS Lett. 2014;588(9):1666–72. https://doi.org/10.1016/j.febslet.2014.02.063.
Vella F, Ferry G, Delagrange P, Boutin JA. NRH:quinone reductase 2: an enzyme of surprises and mysteries. Biochem Pharmacol. 2005;71(1–2):1–12. https://doi.org/10.1016/j.bcp.2005.09.019.
Islam F, Leung KK, Walker MD, Al Massri S, Shilton BH. The unusual cosubstrate specificity of NQO2: conservation throughout the amniotes and implications for cellular function. Front Pharmacol. 2022;13: 838500. https://doi.org/10.3389/fphar.2022.838500.
Yang Y, Zhang N, Zhang G, Sauve AA. NRH salvage and conversion to NAD+ requires NRH kinase activity by adenosine kinase. Nat Metab. 2020;2(4):364–79. https://doi.org/10.1038/s42255-020-0194-9.
Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–43. https://doi.org/10.1126/science.aaf2693.
Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806.
Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016;6:26933. https://doi.org/10.1038/srep26933.
Kim MB, Pham TX, vanLuling M, Kostour V, Kang H, Corvino O, et al. Nicotinamide riboside supplementation exerts an anti-obesity effect and prevents inflammation and fibrosis in white adipose tissue of female diet-induced obesity mice. J Nutr Biochem. 2022;107: 109058. https://doi.org/10.1016/j.jnutbio.2022.109058.
Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–47.
Dollerup OL, Trammell SAJ, Hartmann B, Holst JJ, Christensen B, Møller N, et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab. 2019;104(11):5703–14. https://doi.org/10.1210/jc.2019-01081.
de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–30. https://doi.org/10.1111/acel.12461.
Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26(1):34. https://doi.org/10.1186/s12929-019-0527-8.
Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–36. https://doi.org/10.1016/j.cmet.2011.08.014.
Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, et al. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016;16(7):1851–60. https://doi.org/10.1016/j.celrep.2016.07.027.
Uddin GM, Youngson NA, Sinclair DA, Morris MJ. Head to head comparison of short-term treatment with the NAD(+) precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front Pharmacol. 2016;7:258. https://doi.org/10.3389/fphar.2016.00258.
Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372(6547):1224–9. https://doi.org/10.1126/science.abe9985.
Bushehri N, Jarrell ST, Lieberman S, Mirdamadi-Zonozi N, Birkmayer G, Preuss HG. Oral reduced B-nicotinamide adenine dinucleotide (NADH) affects blood pressure, lipid peroxidation, and lipid profile in hypertensive rats (SHR). Geriatr Nephrol Urol. 1998;8(2):95–100. https://doi.org/10.1023/a:1008242900153.
Roh E, Myoung Kang G, Young Gil S, Hee Lee C, Kim S, Hong D, et al. Effects of chronic NAD supplementation on energy metabolism and diurnal rhythm in obese mice. Obes (Silver Spring). 2018;26(9):1448–56. https://doi.org/10.1002/oby.22263.
Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, et al. nicotinamide improves aspects of healthspan, but not lifespan, in Mice. Cell Metab. 2018;27(3):667-676.e4. https://doi.org/10.1016/j.cmet.2018.02.001.
Alenzi FQ. Effect of nicotinamide on experimental induced diabetes. Iran J Allergy Asthma Immunol. 2009;8(1):11–8.
Barr DP, Russ EM, Eder HA. Protein-lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am J Med. 1951;11(4):480–93. https://doi.org/10.1016/0002-9343(51)90183-0.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham study. Am J Med. 1977;62(5):707–14. https://doi.org/10.1016/0002-9343(77)90874-9.
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. https://doi.org/10.1161/01.cir.79.1.8.
Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem. 1955;54:558–9.
Abdellatif M, Sedej S, Kroemer G. NAD+ metabolism in cardiac health, aging, and disease. Circulation. 2021;144(22):1795–817. https://doi.org/10.1161/CIRCULATIONAHA.
Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13(580):eabd7064. https://doi.org/10.1126/scitranslmed.abd7064.
Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134(12):883–94. https://doi.org/10.1161/CIRCULATIONAHA.116.022495.
Abdellatif M, Bugger H, Kroemer G, Sedej S. NAD+ and vascular dysfunction: from mechanisms to therapeutic opportunities. J Lipid Atheroscler. 2022;11(2):111–32. https://doi.org/10.12997/jla.2022.11.2.111.
Oka SI, Byun J, Huang CY, Imai N, Ralda G, Zhai P, et al. Nampt potentiates antioxidant defense in diabetic cardiomyopathy. Circ Res. 2021;129(1):114–30. https://doi.org/10.1161/CIRCRESAHA.120.317943.
Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105(5):481–91. https://doi.org/10.1161/CIRCRESAHA.109.203703.
Byun J, Oka SI, Imai N, Huang CY, Ralda G, Zhai P, et al. Both gain and loss of Nampt function promote pressure overload-induced heart failure. Am J Physiol Heart Circ Physiol. 2019;317(4):H711–25. https://doi.org/10.1152/ajpheart.00222.2019.
Zeitz MJ, Smyth JW. Translating translation to mechanisms of cardiac hypertrophy. J Cardiovasc Dev Dis. 2020;7(1):9. https://doi.org/10.3390/jcdd7010009.
Pirinen E, Cantó C, Jo YS, Morato L, Zhang H, Menzies KJ, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19(6):1034–41. https://doi.org/10.1016/j.cmet.2014.04.002.
Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. https://doi.org/10.1038/s41467-018-03421-7.
Zhou B, Wang DD, Qiu Y, Airhart S, Liu Y, Stempien-Otero A, et al. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest. 2020;130(11):6054–63. https://doi.org/10.1172/JCI138538.
Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121(3):1163–73.
Kong D, Li J, Shen Y, Liu G, Zuo S, Tao B, et al. Niacin promotes cardiac healing after myocardial infarction through activation of the myeloid prostaglandin D2 receptor subtype 1. J Pharmacol Exp Ther. 2017;360(3):435–44. https://doi.org/10.1124/jpet.116.238261.
D’Andrea E, Hey SP, Ramirez CL, Kesselheim AS. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(4): e192224. https://doi.org/10.1001/jamanetworkopen.2019.2224.
Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–75.
Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–76.
Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, Cohen RA, et al. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med. 2013;45:17–36.
Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE. 2014;9(6): e98972. https://doi.org/10.1371/journal.pone.0098972.
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22(3):401–12. https://doi.org/10.1038/s41593-018-0332-9.
Roca-Agujetas V, de Dios C, Abadin X, Colell A. Upregulation of brain cholesterol levels inhibits mitophagy in Alzheimer disease. Autophagy. 2021;17(6):1555–7. https://doi.org/10.1080/15548627.2021.1920814.
Pradeepkiran JA, Reddy PH. Defective mitophagy in Alzheimer’s disease. Ageing Res Rev. 2020;64: 101191. https://doi.org/10.1016/j.arr.2020.101191.
Reddy PH, Oliver DM. Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in alzheimer’s disease. Cells. 2019;8(5):488. https://doi.org/10.3390/cells8050488.
Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, et al. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc Natl Acad Sci USA. 2021;118(37): e2011226118. https://doi.org/10.1073/pnas.2011226118.
Cen X, Chen Y, Xu X, Wu R, He F, Zhao Q, et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer’s disease mouse model. Nat Commun. 2020;11(1):5731. https://doi.org/10.1038/s41467-020-19547-6.
Chen C, Yang C, Wang J, Huang X, Yu H, Li S, et al. Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer’s disease. J Pineal Res. 2021;71(4): e12774. https://doi.org/10.1111/jpi.12774.
Ghosh N, Das A, Biswas N, Gnyawali S, Singh K, Gorain M, et al. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci Rep. 2020;10(1):20184. https://doi.org/10.1038/s41598-020-76564-7.
Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34(6):1581–8. https://doi.org/10.1016/j.neurobiolaging.2012.12.005.
Love S, Barber R, Wilcock GK. Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain. 1999;122:247–53. https://doi.org/10.1093/brain/122.2.247.
Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA. 2018;115:E1876–85. https://doi.org/10.1073/pnas.1718819115.
Johnson S, Wozniak DF, Imai S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech Dis. 2018;4:10. https://doi.org/10.1038/s41514-018-0029-z.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24: 101192. https://doi.org/10.1016/j.redox.2019.101192.
Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015;15:19. https://doi.org/10.1186/s12883-015-0272-x.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience. 2019;41(5):619–30. https://doi.org/10.1007/s11357-019-00074-2.
Yao Z, Yang W, Gao Z, Jia P. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett. 2017;647:133–40. https://doi.org/10.1016/j.neulet.2017.03.027.
Zhao Y, Guan YF, Zhou XM, Li GQ, Li ZY, Zhou CC, et al. Regenerative neurogenesis after ischemic stroke promoted by nicotinamide phosphoribosyltransferase-nicotinamide adenine dinucleotide cascade. Stroke. 2015;46(7):1966–74. https://doi.org/10.1161/STROKEAHA.115.009216.
Park JH, Long A, Owens K, Kristian T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol Dis. 2016;95:102–10. https://doi.org/10.1016/j.nbd.2016.07.018.
Wei CC, Kong YY, Hua X, Li GQ, Zheng SL, Cheng MH, et al. NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia. Br J Pharmacol. 2017;174(21):3823–36. https://doi.org/10.1111/bph.13979.
Lee HJ, Yang SJ. Supplementation with nicotinamide riboside reduces brain inflammation and improves cognitive function in diabetic mice. Int J Mol Sci. 2019;20(17):4196.
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. https://doi.org/10.1016/S0197-4580(00)00124-X.
Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017;552(7684):187–93. https://doi.org/10.1038/nature25143.
Xie X, Gao Y, Zeng M, Wang Y, Wei TF, Lu YB, et al. Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis. 2019;34(1):353–66. https://doi.org/10.1007/s11011-018-0346-8.
Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA. 2018;115(8):E1876–85. https://doi.org/10.1073/pnas.1718819115.
Burke RE, O’Malley K. Axon degeneration in Parkinson’s disease. Exp Neurol. 2013;246:72–83. https://doi.org/10.1016/j.expneurol.2012.01.011.
Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–91. https://doi.org/10.1126/science.
Vaur P, Brugg B, Mericskay M, Li Z, Schmidt MS, Vivien D, et al. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. 2017;31(12):5440–52. https://doi.org/10.1096/fj.201700221RR.
Brakedal B, Dölle C, Riemer F, Ma Y, Nido GS, Skeie GO, et al. The NADPARK study: a randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 2022;34(3):396–407.
Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD Jr. Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69(3):150–7. https://doi.org/10.1159/000072668.
Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromol Med. 2009;11(1):28–42. https://doi.org/10.1007/s12017-009-8058-1.
Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (Vitamin B(3)) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke. 2000;31(7):1679–85. https://doi.org/10.1161/01.str.31.7.1679.
Wang C, Zhang Y, Ding J, Zhao Z, Qian C, Luan Y, et al. Nicotinamide administration improves remyelination after stroke. Neural Plast. 2017;2017:7019803. https://doi.org/10.1155/2017/7019803.
Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, et al. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–34. https://doi.org/10.2741/2267.
Wang X, Li H, Ding S. The effects of NAD+ on apoptotic neuronal death and mitochondrial biogenesis and function after glutamate excitotoxicity. Int J Mol Sci. 2014;15(11):20449–68. https://doi.org/10.3390/ijms151120449.
Bi J, Li H, Ye SQ, Ding S. Pre-B-cell colony-enhancing factor exerts a neuronal protection through its enzymatic activity and the reduction of mitochondrial dysfunction in in vitro ischemic models. J Neurochem. 2012;120(2):334–46. https://doi.org/10.1111/j.1471-4159.2011.07566.x.
Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39(9):2587–95. https://doi.org/10.1161/STROKEAHA.107.509158.
Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, et al. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28(45):11500–10. https://doi.org/10.1523/JNEUROSCI.3203-08.2008.
Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, et al. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging. 2013;34(6):1564–80. https://doi.org/10.1016/j.neurobiolaging.2012.11.020.
Jia H, Li X, Gao H, Feng Z, Li X, Zhao L, Jia X, Zhang H, Liu J. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res. 2008;86(9):2083–90. https://doi.org/10.1002/jnr.21650.
Caito SW, Aschner M. NAD+ supplementation attenuates methylmercury dopaminergic and mitochondrial toxicity in Caenorhabditis elegans. Toxicol Sci. 2016;151(1):139–49. https://doi.org/10.1093/toxsci/kfw030.
Zheng C, Han J, Xia W, Shi S, Liu J, Ying W. NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett. 2012;512(2):67–71. https://doi.org/10.1016/j.neulet.2012.01.007.
Xie L, Yu S, Wang Z, Yang K, Liu Z, Li C, et al. Nicotinamide adenine dinucleotide protects against spinal cord ischemia reperfusion injury-induced apoptosis by blocking autophagy. Oxid Med Cell Longev. 2017;2017:7063874. https://doi.org/10.1155/2017/7063874.
Huan Q, Sun M, Li M, Zhang D, Han F, Chao WuJ, et al. combination of NAD+ and NADPH offers greater neuroprotection in ischemic stroke models by relieving metabolic stress. Mol Neuro. 2018;55:6063–75. https://doi.org/10.1007/s12035-017-0809-7.
O’Holleran P. DPN in the prevention, diagnosis, and treatment of drug addictions. West J Surg Obst Gyn. 1961;69:213–5.
Mestayer PN. Addiction the dark night of the soul, NAD+ the light of hope. Bloomington: Balboa Press; 2019.
Rex A, Spychalla M, Fink H. Treatment with reduced nicotinamide adenine dinucleotide (NADH) improves water maze performance in old Wistar rats. Behav Brain Res. 2004;154(1):149–53. https://doi.org/10.1016/j.bbr.2004.02.001.
Dunbar RL, Gelfand JM. Seeing red: flushing out instigators of niacin-associated skin toxicity. J Clin Invest. 2010;120:2651–5.
Kamanna VS, Kashyap ML. Nicotinic acid (niacin) receptor agonists: will they be useful therapeutic agents? Am J Cardiol. 2007;100(11A):S53-61. https://doi.org/10.1016/j.amjcard.2007.09.080.
Lv H, Lv G, Chen C, Zong Q, Jiang G, Ye D, et al. NAD+ metabolism maintains inducible PD-L1 expression to drive tumor immune evasion. Cell Metab. 2021;33(1):110–27.
Navas LE, Carnero A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct Target Ther. 2021;6(1):2. https://doi.org/10.1038/s41392-020-00354-w.
Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6(10): e918. https://doi.org/10.1038/tp.2016.200.
Grant R, Berg J, Mestayer R, Braidy N, Bennett J, Broom S, et al. A pilot study investigating changes in the human plasma and urine NAD+ metabolome during a 6 hour intravenous infusion of NAD. Front Aging Neurosci. 2019;11:257. https://doi.org/10.3389/fnagi.2019.00257.
Anti-aging Services Market Size, Share & Trends Analysis Report By Demographics, By Type (Chemical Peel, BOTOX, Microdermabrasion, Breast Augmentation, Liposuction), And Segment Forecasts, 2019–2026. Grandview Research; 2019. p. GVR-2-68038-815-2.
Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (Nicotinamide Riboside Chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep. 2019;9(1):9772. https://doi.org/10.1038/s41598-019-46120-z.
Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. https://doi.org/10.1038/ncomms12948.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
NB is a recipient of the NHMRC Emerging Leadership EL2 Award at the Centre for Healthy Brain Ageing, University of New South Wales, Sydney, Australia.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Author contributions
TH and NB wrote and revised the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Helman, T., Braidy, N. Importance of NAD+ Anabolism in Metabolic, Cardiovascular and Neurodegenerative Disorders. Drugs Aging 40, 33–48 (2023). https://doi.org/10.1007/s40266-022-00989-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-00989-0