Abstract
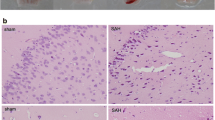
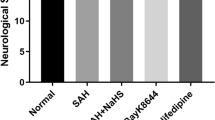
Early brain injury (EBI) is the leading cause of poor prognosis for patients suffering from subarachnoid hemorrhage (SAH), particularly learning and memory deficits in the repair phase. A recent report has involved calcium/calmodulin-dependent protein kinase II (CaMKII) in the pathophysiological process underlying SAH-induced EBI. Alpha-asarone (ASA), a major compound isolated from the Chinese medicinal herb Acorus tatarinowii Schott, was proven to reduce secondary brain injury by decreasing CaMKII over-phosphorylation in rats’ model of intracerebral hemorrhage in our previous report. However, the effect of ASA on SAH remains unclear, and the role of CaMKII in both acute and recovery stages of SAH needs further investigation. In this work, we first established a classic SAH rat model by endovascular perforation and intraperitoneally administrated different ASA doses (10, 20, and 40 mg/kg) 2 h after successful modeling. Then, the short- and long-term neurobehavioral performances were blindly evaluated to confirm ASA’s efficacy against SAH. Subsequently, we explored ASA’s therapeutic mechanism in both acute and recovery stages using histopathological examination, TUNEL staining, flow cytometry, Western-blot, double-immunofluorescence staining, and transmission electron microscopy (TEM) observation. Finally, KN93, a selective CaMKII inhibitor, was applied in oxyhemoglobin-damaged HT22 cells to explore the role of CaMKII in ASA’s neuroprotective effect. The results demonstrated that ASA alleviated short- and long-term neurological dysfunction, reduced mortality and seizure rate within 24 h, and prolonged 14-day survival in SAH rats. Histopathological examination showed a reduction of neuronal damage and a restoration of the hippocampal structure after ASA treatment in both acute and recovery phases of SAH. In the acute stage, the Western-blot and flow cytometer analyses showed that ASA restored E/I balance, reduced calcium overload and CaMKII phosphorylation, and inhibited mitochondrion-involved apoptosis, thus preventing neuronal damage and apoptosis underlying EBI post-SAH. In the recovery stage, the TEM observation, double-immunofluorescence staining, and Western-blot analyses indicated that ASA increased the numbers of synapses and enhanced synaptic plasticity in the ipsilateral hippocampi, probably by promoting NR2B/CaMKII interaction and activating subsequent CREB/BDNF/TrkB signaling pathways. Furthermore, KN93 notably reversed ASA’s neuroprotective effect on oxyhemoglobin-damaged HT22 cells, confirming CaMKII a potential target for ASA’s efficacy against SAH. Our study confirmed for the first time that ASA ameliorated the SAH rats’ neurobehavioral deterioration, possibly via modulating CaMKII-involved pathways. These findings provided a promising candidate for the clinical treatment of SAH and shed light on future drug discovery against SAH.










Similar content being viewed by others

Data Availability
Data from this study are available from the corresponding author on reasonable request.
References
Chen F, Lu J, Chen F, Lin Z, Lin Y, Yu L, Su X, Yao P, Cai B, Kang D. Recombinant neuroglobin ameliorates early brain injury after subarachnoid hemorrhage via inhibiting the activation of mitochondria apoptotic pathway. Neurochem Int. 2018;112:219–26.
Nath S, Koziarz A, Badhiwala JH, Almenawer SA. Predicting outcomes in aneurysmal subarachnoid haemorrhage. BMJ. 2018;360:k102.
Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76:588–97.
Li J, Chen J, Mo H, Chen J, Qian C, Yan F, Gu C, Hu Q, Wang L, Chen G. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol. 2016;53:2668–78.
Luo X, Li L, Xu W, Cheng Y, Xie Z. HLY78 attenuates neuronal apoptosis via the LRP6/GSK3β/β-Catenin signaling pathway after subarachnoid hemorrhage in rats. Neurosci Bull. 2020;36:1171–81.
Feng D, Wang W, Dong Y, Wu L, Huang J, Ma Y, Zhang Z, Wu S, Gao G, Qin H. Ceftriaxone alleviates early brain injury after subarachnoid hemorrhage by increasing excitatory amino acid transporter 2 expression via the PI3K/Akt/NF-κB signaling pathway. Neuroscience. 2014;268:21–32.
Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–81.
Wu CT, Wen LL, Wong CS, Tsai SY, Chan SM, Yeh CC, Borel CO, Cherng CH. Temporal changes in glutamate, glutamate transporters, basilar arteries wall thickness, and neuronal variability in an experimental rat model of subarachnoid hemorrhage. Anesth Analg. 2011;112:666–73.
Geraghty JR, Lara-Angulo MN, Spegar M, Reeh J, Testai FD. Severe cognitive impairment in aneurysmal subarachnoid hemorrhage: predictors and relationship to functional outcome. J Stroke Cerebrovasc Dis. 2020;29:105027.
Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72.
Shibata ACE, Ueda HH, Eto K, Onda M, Sato A, Ohba T, Nabekura J, Murakoshi H. Photoactivatable CaMKII induces synaptic plasticity in single synapses. Nat Commun. 2021;12:751.
Li Z, Zhao G, Qian S, Yang Z, Chen X, Chen J, Cai C, Liang X, Guo J. Cerebrovascular protection of β-asarone in Alzheimer’s disease rats: a behavioral, cerebral blood flow, biochemical and genic study. J Ethnopharmacol. 2012;144:305–12.
Rajput SB, Tonge MB, Karuppayil SM. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine. 2014;21:268–76.
Chellian R, Pandy V, Mohamed Z. Pharmacology and toxicology of α- and β-asarone: a review of preclinical evidence. Phytomedicine. 2017;32:41–58.
Chen Y, Gao X, Liu Q, Zeng L, Zhang K, Mu K, Zhang D, Zou H, Wu N, Ou J, Wang Q, Mao S. Alpha-asarone improves cognitive function of aged rats by alleviating neuronal excitotoxicity via GABA(A) receptors. Neuropharmacology. 2020;162:107843.
Wang ZJ, Levinson SR, Sun L, Heinbockel T. Identification of both GABAA receptors and voltage-activated Na(+) channels as molecular targets of anticonvulsant α-asarone. Front Pharmacol. 2014;5:40.
Zhang K, Liu Q, Luo L, Feng X, Hu Q, Fan X, Mao S. Neuroprotective effect of alpha-asarone on the rats model of cerebral ischemia-reperfusion stroke via ameliorating glial activation and autophagy. Neuroscience. 2021;473:130–41.
Gao X, Li R, Luo L, Zhang D, Liu Q, Zhang J, Mao S. Alpha-asarone ameliorates neurological deterioration of intracerebral hemorrhagic rats by alleviating secondary brain injury via anti-excitotoxicity pathways. Phytomedicine. 2022;105:154363.
Ma WC, Zhang Q, Li H, Larregieu CA, Zhang N, Chu T, Jin H, Mao SJ. Development of intravenous lipid emulsion of α-asarone with significantly improved safety and enhanced efficacy. Int J Pharm. 2013;450:21–30.
Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–34.
Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94.
Xie Z, Enkhjargal B, Wu L, Zhou K, Sun C, Hu X, Gospodarev V, Tang J, You C, Zhang JH. Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology. 2018;128:142–51.
Possin KL, Sanchez PE, Anderson-Bergman C, Fernandez R, Kerchner GA, Johnson ET, Davis A, Lo I, Bott NT, Kiely T, Fenesy MC, Miller BL, Kramer JH, Finkbeiner S. Cross-species translation of the Morris maze for Alzheimer’s disease. J Clin Invest. 2016;126:779–83.
Li JR, Xu HZ, Nie S, Peng YC, Fan LF, Wang ZJ, Wu C, Yan F, Chen JY, Gu C, Wang C, Chen JS, Wang L, Chen G. Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats. J Neuroinflammation. 2017;14:186.
Xiang H, Zhang Q, Han Y, Yang L, Zhang Y, Liu Q, Zhang Z, Zhang L. Novel brain-targeting 3-n-butylphthalide prodrugs for ischemic stroke treatment. J Control Release. 2021;335:498–514.
Nakao T, Horie T, Baba O, Nishiga M, Nishino T, Izuhara M, Kuwabara Y, Nishi H, Usami S, Nakazeki F, Ide Y, Koyama S, Kimura M, Sowa N, Ohno S, Aoki H, Hasegawa K, Sakamoto K, Minatoya K, Kimura T, Ono K. Genetic ablation of microRNA-33 attenuates inflammation and abdominal aortic aneurysm formation via several anti-inflammatory pathways. Arterioscler Thromb Vasc Biol. 2017;37:2161–70.
Kong LH, Gu XM, Wu F, Jin ZX, Zhou JJ. CaMKII inhibition mitigates ischemia/reperfusion-elicited calpain activation and the damage to membrane skeleton proteins in isolated rat hearts. Biochem Biophys Res Commun. 2017;491:687–92.
J. Lu, L. Wu, X. Wang, J. Zhu, J. Du, B. Shen. Detection of mitochondria membrane potential to study CLIC4 knockdown-induced HN4 cell apoptosis in vitro. J Vis Exp 2018;56317.
Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–91.
Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–53.
Chen Y, Huang L, Wang L, Chen L, Ren W, Zhou W. Differential expression of microRNAs contributed to the health efficacy of EGCG in in vitro subarachnoid hemorrhage model. Food Funct. 2017;8:4675–83.
Zong P, Feng J, Yue Z, Li Y, Wu G, Sun B, He Y, Miller B, Yu AS, Su Z, Xie J, Mori Y, Hao B, Yue L. Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury. Neuron. 2022;110:1944-1958.e8.
Yuksel S, Tosun YB, Cahill J, Solaroglu I. Early brain injury following aneurysmal subarachnoid hemorrhage: emphasis on cellular apoptosis. Turk Neurosurg. 2012;22:529–33.
Sun JY, Zhao SJ, Wang HB, Hou YJ, Mi QJ, Yang MF, Yuan H, Ni QB, Sun BL, Zhang ZY. Ifenprodil improves long-term neurologic deficits through antagonizing glutamate-induced excitotoxicity after experimental subarachnoid hemorrhage. Transl Stroke Res. 2021;12:1067–80.
Clarkson AN, Chebib M. The role of peri-synaptic GABA receptors after stroke. In: Errington AC, Di Giovanni G, Crunelli V, editors. Extrasynaptic GABAA receptors. New York: Springer; 2014. p. 179–205.
Zhao YM, Yu SM, Zhou QK. Effect of baicalin on the brain expression of GAT-1 and GABAAR after intracerebral hemorrhage in rats. Chin New Drugs J. 2010;19:970–4.
Soni N, Reddy BV, Kumar P. GLT-1 transporter: an effective pharmacological target for various neurological disorders. Pharmacol Biochem Behav. 2014;127:70–81.
Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–88.
Moriguchi S, Inagaki R, Saito T, Saido TC, Fukunaga K. Propolis promotes memantine-dependent rescue of cognitive deficits in APP-KI mice. Mol Neurobiol. 2022;59:4630–46.
Song Q, Fan C, Wang P, Li Y, Yang M, Yu SY. Hippocampal CA1 βCaMKII mediates neuroinflammatory responses via COX-2/PGE2 signaling pathways in depression. J Neuroinflammation. 2018;15:338.
Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett. 2011;585:921–6.
Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–78.
Tait SWG, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013;5:a008706.
ZakiGhali MG, Srinivasan VM, Wagner K, Rao C, Chen SR, Johnson JN, Kan P. Cognitive sequelae of unruptured and ruptured intracranial aneurysms and their treatment: modalities for neuropsychological assessment. World Neurosurg. 2018;120:537–49.
Luo Z. Synapse formation and remodeling. Sci China Life Sci. 2010;53:315–21.
Yang C, Li T, Xue H, Wang L, Deng L, Xie Y, Bai X, Xin D, Yuan H, Qiu J, Wang Z, Li G. Inhibition of necroptosis rescues SAH-induced synaptic impairments in hippocampus via CREB-BDNF pathway. Front Neurosci. 2018;12:990.
Perrone-Capano C, Volpicelli F, Penna E, Chun JT, Crispino M. Presynaptic protein synthesis and brain plasticity: from physiology to neuropathology. Prog Neurobiol. 2021;202:102051.
Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays. 2004;26:445–53.
Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc Natl Acad Sci U S A. 2015;112:E6983-92.
Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41.
Plata A, Lebedeva A, Denisov P, Nosova O, Postnikova TY, Pimashkin A, Brazhe A, Zaitsev AV, Rusakov DA, Semyanov A. Astrocytic atrophy following status epilepticus parallels reduced Ca2+ activity and impaired synaptic plasticity in the rat hippocampus. Front Mol Neurosci. 2018;11:215.
Incontro S, Díaz-Alonso J, Iafrati J, Vieira M, Asensio CS, Sohal VS, Roche KW, Bender KJ, Nicoll RA. The CaMKII/NMDA receptor complex controls hippocampal synaptic transmission by kinase-dependent and independent mechanisms. Nat Commun. 2018;9:2069.
Xie W, Meng X, Zhai Y, Ye T, Zhou P, Nan F, Sun G, Sun X. Antidepressant-like effects of the Guanxin Danshen formula via mediation of the CaMK II-CREB-BDNF signalling pathway in chronic unpredictable mild stress-induced depressive rats. Ann Transl Med. 2019;7:564.
Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76 Pt C:639–56.
Wong MH, Samal AB, Lee M, Vlach J, Novikov N, Niedziela-Majka A, Feng JY, Koltun DO, Brendza KM, Kwon HJ, Schultz BE, Sakowicz R, Saad JS, Papalia GA. The KN-93 molecule inhibits calcium/calmodulin-dependent protein kinase II (CaMKII) activity by binding to Ca(2+)/CaM. J Mol Biol. 2019;431:1440–59.
Author information
Authors and Affiliations
Contributions
Xiaofeng Gao and Shengjun Mao designed the study. Xiaofeng Gao and Rui Li performed the in vivo and in vitro pharmacological experiments in SAH rats. Lijun Luo, Can Liao, and Huiyuan Yang carried out the data acquisition, analysis, and interpretation. The manuscript was drafted by Xiaofeng Gao and revised by Shengjun Mao. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, X., Li, R., Luo, L. et al. Alpha-Asarone Ameliorates Neurological Dysfunction of Subarachnoid Hemorrhagic Rats in Both Acute and Recovery Phases via Regulating the CaMKII-Dependent Pathways. Transl. Stroke Res. 15, 476–494 (2024). https://doi.org/10.1007/s12975-023-01139-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-023-01139-3