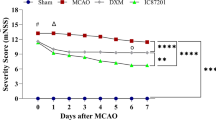
Abstract
The blood–brain barrier (BBB) disruption leads to the vasogenic brain edema and contributes to the early brain injury (EBI) after subarachnoid hemorrhage (SAH). However, the mechanisms underlying the BBB damage following SAH are poorly understood. Here we reported that the neurotransmitter glutamate of cerebrospinal fluid (CSF) was dramatically increased in SAH patients with symptoms of cerebral edema. Using the rat SAH model, we found that SAH caused the increase of CSF glutamate level and BBB permeability in EBI, intracerebroventricular injection of exogenous glutamate deteriorated BBB damage and cerebral edema, while intraperitoneally injection of metabotropic glutamate receptor 1(mGluR1) negative allosteric modulator JNJ16259685 significantly attenuated SAH-induced BBB damage and cerebral edema. In an in vitro BBB model, we showed that glutamate increased monolayer permeability of human brain microvascular endothelial cells (HBMEC), whereas JNJ16259685 preserved glutamate-damaged BBB integrity in HBMEC. Mechanically, glutamate downregulated the level and phosphorylation of vasodilator-stimulated phosphoprotein (VASP), decreased the tight junction protein occludin, and increased AQP4 expression at 72 h after SAH. However, JNJ16259685 significantly increased VASP, p-VASP, and occludin, and reduced AQP level at 72 h after SAH. Altogether, our results suggest an important role of glutamate in disruption of BBB function and inhibition of mGluR1 with JNJ16259685 reduced BBB damage and cerebral edema after SAH.







Similar content being viewed by others
References
Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97(1):14–37. https://doi.org/10.1016/j.pneurobio.2012.02.003.
Duris K, Lipkova J, Splichal Z, Madaraszova T, Jurajda M. Early inflammatory response in the brain and anesthesia recovery time evaluation after experimental subarachnoid hemorrhage. Transl Stroke Res. 2018;10:308–18. https://doi.org/10.1007/s12975-018-0641-z.
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014;115:64–91. https://doi.org/10.1016/j.pneurobio.2013.09.002.
Li Z, Liang G, Ma T, Li J, Wang P, Liu L, et al. Blood-brain barrier permeability change and regulation mechanism after subarachnoid hemorrhage. Metab Brain Dis. 2015;30(2):597–603. https://doi.org/10.1007/s11011-014-9609-1.
Kanamaru H, Suzuki H. Potential therapeutic molecular targets for blood-brain barrier disruption after subarachnoid hemorrhage. Neural Regen Res. 2019;14(7):1138–43. https://doi.org/10.4103/1673-5374.251190.
Nag S, Manias JL, Stewart DJ. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009;118(2):197–217. https://doi.org/10.1007/s00401-009-0541-0.
Hayman EG, Wessell A, Gerzanich V, Sheth KN, Simard JM. Mechanisms of global cerebral edema formation in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26(2):301–10. https://doi.org/10.1007/s12028-016-0354-7.
Ivanidze J, Ferraro RA, Giambrone AE, Segal AZ, Gupta A, Sanelli PC. Blood-brain barrier permeability in aneurysmal subarachnoid hemorrhage: correlation with clinical outcomes. AJR Am J Roentgenol. 2018;211(4):891–5. https://doi.org/10.2214/AJR.17.18237.
Huang S, Cao J, Jiang M, Labesse G, Liu J, Pin JP, et al. Interdomain movements in metabotropic glutamate receptor activation. Proc Natl Acad Sci U S A. 2011;108(37):15480–5. https://doi.org/10.1073/pnas.1107775108.
Vazana U, Veksler R, Pell GS, Prager O, Fassler M, Chassidim Y, et al. Glutamate-mediated blood-brain barrier opening: implications for neuroprotection and drug delivery. J Neurosci. 2016;36(29):7727–39. https://doi.org/10.1523/JNEUROSCI.0587-16.2016.
Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, et al. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J Biol Chem. 2002;277(17):14801–11. https://doi.org/10.1074/jbc.M110557200.
Sharp CD, Hines I, Houghton J, Warren A, Jackson TH, Jawahar A, et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol Heart Circ Physiol. 2003;285(6):H2592–8. https://doi.org/10.1152/ajpheart.00520.2003.
Andras IE, Deli MA, Veszelka S, Hayashi K, Hennig B, Toborek M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab. 2007;27(8):1431–43. https://doi.org/10.1038/sj.jcbfm.9600445.
Jung CS, Lange B, Zimmermann M, Seifert V. CSF and serum biomarkers focusing on cerebral vasospasm and ischemia after subarachnoid hemorrhage. Stroke Res Treat. 2013;2013:560305. https://doi.org/10.1155/2013/560305.
Jacobsen A, Nielsen TH, Nilsson O, Schalen W, Nordstrom CH. Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol Scand. 2014;130(3):156–63. https://doi.org/10.1111/ane.12258.
Westermaier T, Jauss A, Eriskat J, Kunze E, Roosen K. The temporal profile of cerebral blood flow and tissue metabolites indicates sustained metabolic depression after experimental subarachnoid hemorrhage in rats. Neurosurgery. 2011;68(1):223–9; discussion 9-30. https://doi.org/10.1227/NEU.0b013e3181fe23c1.
Rostami E, Engquist H, Howells T, Johnson U, Ronne-Engstrom E, Nilsson P, et al. Early low cerebral blood flow and high cerebral lactate: prediction of delayed cerebral ischemia in subarachnoid hemorrhage. J Neurosurg. 2018;128(6):1762–70. https://doi.org/10.3171/2016.11.JNS161140.
Sokol B, Urbaniak B, Wasik N, Plewa S, Klupczynska A, Jankowski R, et al. Amino acids in cerebrospinal fluid of patients with aneurysmal subarachnoid haemorrhage: an observational study. Front Neurol. 2017;8:438. https://doi.org/10.3389/fneur.2017.00438.
Zhang Z, Liu J, Fan C, Mao L, Xie R, Wang S, et al. The GluN1/GluN2B NMDA receptor and metabotropic glutamate receptor 1 negative allosteric modulator has enhanced neuroprotection in a rat subarachnoid hemorrhage model. Exp Neurol. 2018;301(Pt A):13–25. https://doi.org/10.1016/j.expneurol.2017.12.005.
Wang W, Han P, Xie R, Yang M, Zhang C, Mi Q, et al. TAT-mGluR1 attenuation of neuronal apoptosis through prevention of MGluR1alpha truncation after experimental subarachnoid hemorrhage. ACS Chem Neurosci. 2019;10(1):746–56. https://doi.org/10.1021/acschemneuro.8b00531.
Lavreysen H, Wouters R, Bischoff F, Nobrega Pereira S, Langlois X, Blokland S, et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47(7):961–72. https://doi.org/10.1016/j.neuropharm.2004.08.007.
Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. https://doi.org/10.1146/annurev.pharmtox.011008.145533.
Liu J, Zhang Z, Moreno-Delgado D, Dalton JA, Rovira X, Trapero A, et al. Allosteric control of an asymmetric transduction in a G protein-coupled receptor heterodimer. eLife. 2017;6. https://doi.org/10.7554/eLife.26985.
Sarrafzadeh A, Haux D, Sakowitz O, Benndorf G, Herzog H, Kuechler I, et al. Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage: relation of clinical course, CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke. 2003;34(6):1382–8. https://doi.org/10.1161/01.STR.0000074036.97859.02.
Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167(2):327–34. https://doi.org/10.1016/j.jneumeth.2007.08.004.
Zhang ZY, Sun BL, Liu JK, Yang MF, Li DW, Fang J, et al. Activation of mGluR5 attenuates microglial activation and neuronal apoptosis in early brain injury after experimental subarachnoid hemorrhage in rats. Neurochem Res. 2015;40(6):1121–32. https://doi.org/10.1007/s11064-015-1572-7.
Zhang ZY, Jiang M, Fang J, Yang MF, Zhang S, Yin YX, et al. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Mol Neurobiol. 2017;54(1):1–14. https://doi.org/10.1007/s12035-015-9635-y.
Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain. 2017;140(7):1914–31. https://doi.org/10.1093/brain/awx111.
Sarrafzadeh AS, Sakowitz OW, Kiening KL, Benndorf G, Lanksch WR, Unterberg AW. Bedside microdialysis: a tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit Care Med. 2002;30(5):1062–70.
Samuelsson C, Hillered L, Zetterling M, Enblad P, Hesselager G, Ryttlefors M, et al. Cerebral glutamine and glutamate levels in relation to compromised energy metabolism: a microdialysis study in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2007;27(7):1309–17. https://doi.org/10.1038/sj.jcbfm.9600433.
Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33(5):1225–32.
Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002;16(6):583–5. https://doi.org/10.1096/fj.01-0739fje.
Reinhard M, Halbrugge M, Scheer U, Wiegand C, Jockusch BM, Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992;11(6):2063–70.
Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53(3):399–412. https://doi.org/10.1016/j.neuron.2006.12.020.
Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011;63(1):35–58. https://doi.org/10.1124/pr.110.004036.
Gillard SE, Tzaferis J, Tsui HC, Kingston AE. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J Comp Neurol. 2003;461(3):317–32. https://doi.org/10.1002/cne.10671.
Garzon-Muvdi T, Pradilla G, Ruzevick JJ, Bender M, Edwards L, Grossman R, et al. A glutamate receptor antagonist, S-4-carboxyphenylglycine (S-4-CPG), inhibits vasospasm after subarachnoid hemorrhage in haptoglobin 2-2 mice [corrected]. Neurosurgery. 2013;73(4):719–28; discussion 29. https://doi.org/10.1227/NEU.0000000000000080.
Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14(4):265–77. https://doi.org/10.1038/nrn3468.
Tait MJ, Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience. 2010;167(1):60–7. https://doi.org/10.1016/j.neuroscience.2010.01.053.
Cao S, Zhu P, Yu X, Chen J, Li J, Yan F, et al. Hydrogen sulfide attenuates brain edema in early brain injury after subarachnoid hemorrhage in rats: possible involvement of MMP-9 induced blood-brain barrier disruption and AQP4 expression. Neurosci Lett. 2016;621:88–97. https://doi.org/10.1016/j.neulet.2016.04.018.
Qi W, Cao D, Li Y, Peng A, Wang Y, Gao K, et al. Atorvastatin ameliorates early brain injury through inhibition of apoptosis and ER stress in a rat model of subarachnoid hemorrhage. Biosci Rep. 2018;38(3). https://doi.org/10.1042/BSR20171035.
Badaut J, Brunet JF, Grollimund L, Hamou MF, Magistretti PJ, Villemure JG, et al. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir Suppl. 2003;86:495–8.
Saadoun S, Papadopoulos MC, Krishna S. Water transport becomes uncoupled from K+ siphoning in brain contusion, bacterial meningitis, and brain tumours: immunohistochemical case review. J Clin Pathol. 2003;56(12):972–5. https://doi.org/10.1136/jcp.56.12.972.
Gunnarson E, Zelenina M, Axehult G, Song Y, Bondar A, Krieger P, et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia. 2008;56(6):587–96. https://doi.org/10.1002/glia.20627.
Shi Z, Zhang W, Lu Y, Lu Y, Xu L, Fang Q, et al. Aquaporin 4-mediated glutamate-induced astrocyte swelling is partially mediated through metabotropic glutamate receptor 5 activation. Front Cell Neurosci. 2017;11:116. https://doi.org/10.3389/fncel.2017.00116.
Funding
This work was supported by funds from the National Natural Science Foundation of China (Grant No. 81671141 and 81870938), the Taishan Scholars Project (to Bao-liang Sun), the Shanghai Science and Technology Commission (19431903200 to Ming Jiang), and the Youth Innovation Team of Shandong Universities (2019KJK001 to Zong-yong Zhang).
Author information
Authors and Affiliations
Contributions
BS and ZZ designed the experiments. CZ, JM, WW, SZ, YY, QM, YS, and MY performed the experiments. ZZ and BS analyzed the results. ZZ wrote the manuscript with contribution from BS. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, C., Jiang, M., Wang, Wq. et al. Selective mGluR1 Negative Allosteric Modulator Reduces Blood–Brain Barrier Permeability and Cerebral Edema After Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 11, 799–811 (2020). https://doi.org/10.1007/s12975-019-00758-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00758-z