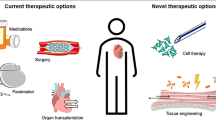
Abstract
Chronic brain injury following cerebral ischemia is a severe debilitating neurological condition, where clinical intervention is well known to decrease morbidity and mortality. Despite the development of several therapeutic strategies, clinical outcome in the majority of patients could be better improved, since many still face life-long neurological deficits. Among the several strategic options that are currently being pursued, tissue engineering provides much promise for neural tissue salvage and regeneration in brain ischemia. Specifically, hydrogel biomaterials have been utilized to docket biomolecules, adhesion motifs, growth factors, and other proneural cues for stable stem cell encapsulation. Here, we provide an overview of therapeutic applications of hydrogels in stroke treatment. Special focus is given to design considerations for generation of efficient hydrogel systems for neurological applications. Therapeutic applications of hydrogels in stroke as conducive microenvironments for stem cell transplantation and drug delivery have been discussed. Finally, we present our perspectives on clinical translation of hydrogels for neural tissue regeneration.

Similar content being viewed by others
Abbreviations
- BBB :
-
Blood blood-brain barrier
- 3D :
-
Three-dimensional
- CNS :
-
Central nervous system
- ECM :
-
Extracellular matrix
- HA :
-
Hyaluronic acid
- GAG :
-
Glycoseaminoglycans
- ECF :
-
Extracellular fluid
- PEG :
-
poly(ethylene glycol)
- N-CAM :
-
Neural cell adhesion molecule
- hNPCs :
-
Human neural progenitor cells
- MMP :
-
Matrix metalloproteinase
- BMP-4 :
-
Bone morphogenic protein-4
- BDNF :
-
Brain-derived neurotrophic factor
- iPSCs :
-
Induced pluripotent stem cells
- iPSC-NPCs :
-
iPSC-derived NPCs
- RGD :
-
Arginine-glycine-aspartic acid
- ELPs :
-
Elastin-like peptides
- NSC :
-
Neural stem cell
- hPSC :
-
Human pluripotent stem cell
- hUVECs :
-
Human umbilical vein endothelial cells
- CAM :
-
Chorioallantoic membrane
- E :
-
Elastic modulus
- NSPCs :
-
Neural stem and progenitor cells
- MAC :
-
Methacrylamide chitosan
- UBM :
-
Urinary bladder matrix
- EGF :
-
Epidermal growth factor
- PPy :
-
polypyrrole
- VEGF :
-
Vascular endothelial growth factor
- HepMA :
-
Heparin methacrylate
- PVA :
-
poly(vinyl alcohol)
- PEDOT :
-
poly(3,4-ethylenedioxythiophene)
- pTS :
-
paratoluenesulfonate
- PANI :
-
polyaniline
- p(GMA) :
-
poly(glyceryl methacrylate)
- PHPMA :
-
poly(N-2-hydroxypropyl methacrylamide)
- PEGDA :
-
poly(ethyleneglycol diacrylate)
- LCST :
-
Lower critical solution temperature
- UCST :
-
Upper critical solution temperature
- pNIPAAm :
-
poly(N-isopropylacrylamide)
- PLLA :
-
poly-l-(lactic acid)
- PEO-PPO-PEO :
-
poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide)
- pNIPAAm-PEG :
-
pNIPAAm grafted PEG
- PUASM :
-
poly(urethane amino sulfamethazine)
- S-NBC :
-
S-(2-nitrobenzyl)cysteine)
- GRGDS :
-
Gly-Arg-Gly-Asp-Ser
- EPO :
-
Erythropoietin
- G-CSF :
-
Granulocyte colony-stimulating factor
- HAMC :
-
Hyaluronan/methyl cellulose
- PLGA :
-
poly(lactic-co-glycolic acid)
- PDMS-TEOS :
-
polydimethysiloxane-tetraethoxysilane
- Ang-1 :
-
Angiopoietin-1
- ESC :
-
Embryonic stem cell
- PCL :
-
polycaprolactone
- GSH :
-
Genipin cross-linked sericin hydrogel
- hEnSCs :
-
Human endometrial stem cells
- SCs :
-
Schwann cells
- PFTBA :
-
perfluorotributylamine
- MRI :
-
Magnetic resonance imaging
- PET :
-
Positron emission tomography
- BLI :
-
Bioluminescence imaging
- CEST:
-
Chemical exchange saturation transfer
- GLP-1 :
-
Glucagon-like peptide-1
- STEP :
-
Striatal enriched tyrosine phosphatase
- TBI :
-
Traumatic brain injury
- hUC-MSCs :
-
Human umbilical cord mesenchymal stem cells
- SDF-1α :
-
Stromal cell-derived factor 1α
- CS-GAG :
-
Chondroitin sulfate glycosaminoglycan
- SCI :
-
Spinal cord injury
- NGF :
-
Nerve growth factor
- hUTCs :
-
Human umbilical tissue-derived cells
- VM :
-
Ventral midbrain
- DA :
-
Dopamine
- BDNF-His :
-
Hexahistidine peptide-linked recombinant BDNF
- PD :
-
Parkinson’s disease
- PAA :
-
poly(amidoamine)
- TH :
-
Tyrosine hydroxylase
- GBM :
-
Glioblastoma
- GemC12 :
-
Gemcitabine
- ICG :
-
Indocyanine green
- PEG-DMA :
-
polyethylene glycol dimethacrylate
- TMZ :
-
Temozolomide
- PC gel :
-
poly(ethylene glycol)-g-chitosan hydrogel
References
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:146–603.
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–48.
Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23.
Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111:483–95.
Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98.
Thomas B, Julien B. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–22.
Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS. Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology. 2014;39:169–88.
Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–95.
Zhang P, Lei X, Sun Y, Zhang H, Chang L, Li C, et al. Regenerative repair of pifithrin-α in cerebral ischemia via VEGF dependent manner. Sci Rep. 2016;6:1–10.
Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cereb Blood Flow Metab. 2012;32:1317–31.
Chan SJ, Love C, Spector M, Cool SM, Nurcombe V, Lo EH. Endogenous regeneration: engineering growth factors for stroke. Neurochem Int. 2017;107:57–65.
Cooke MJ, Wang Y, Morshead CM, Shoichet MS. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials. 2011;32:5688–97.
Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84.
Tang YH, Ma YY, Zhang ZJ, Wang YT, Yang GY. Opportunities and challenges: stem cell-based therapy for the treatment of ischemic stroke. CNS Neurosci Ther. 2015;21:337–47.
Wang J, Yang W, Xie H, Song Y, Li Y, Wang L. Ischemic stroke and repair: current trends in research and tissue engineering treatments. Regen Med Res. 2014;2:1–10.
Babita Mahanta RN. An overview of various biomimetic scaffolds: challenges and applications in tissue engineering. J Tissue Sci Eng. 2014;5:1–5.
Osathanon T, Linnes ML, Rajachar RM, Ratner BD, Somerman MJ, Giachelli CM. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. 2008;29:4091–9.
Kumbar SG, Toti US, Deng M, James R, Laurencin CT, Aravamudhan A, et al. Novel mechanically competent polysaccharide scaffolds for bone tissue engineering. Biomed Mater. 2011;6:1–13.
Florine EM, Miller RE, Liebesny PH, Mroszczyk KA, Lee RT, Patwari P, et al. Delivering heparin-binding insulin-like growth factor 1 with self-assembling peptide hydrogels. Tissue Eng A. 2015;21:637–46.
Shin YC, Kim J, Kim SE, Song SJ, Hong SW, Oh JW, et al. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regen Biomater. 2017;4:159–66.
Somaa FA, Wang TY, Niclis JC, Bruggeman KF, Kauhausen JA, Guo H, et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017;20:1964–77.
Wang J, Chen F, Liu L, Qi C, Wang B, Yan X, et al. Engineering EMT using 3D micro-scaffold to promote hepatic functions for drug hepatotoxicity evaluation. Biomaterials. 2016;91:11–22.
Srinivasan S, Jayasree R, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr Polym. 2012;87:274–83.
Mohtaram NK, Karamzadeh V, Shafieyan Y, Willerth SM. Commercializing electrospun scaffolds for pluripotent stem cell-based tissue engineering applications. Gruyter Open. 2017;2:62–72.
Qi C, Sun T. Comparative study of porous hydroxyapatite / chitosan and whitlockite / chitosan scaffolds for bone regeneration in calvarial defects. Int J Nanomedicine. 2017;12:2673–87.
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6.
Garfein ES, Orgill DP, Pribaz JJ. Clinical applications of tissue engineered constructs. Clin Plast Surg. 2003;30:485–98.
Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg. 2011;92:1308–14.
Khouri RK, Rigotti G, Cardoso E, Marchi A, Rotemberg SC, Baker TJ, et al. Tissue-engineered breast reconstruction with brava-assisted fat grafting: a 7-year, 488-patient, multicenter experience. Plast Reconstr Surg. 2015;135:643–58.
Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan A, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30.
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47.
Mdzinarishvili A, Sutariya V, Talasila PK, Geldenhuys WJ, Sadana P. Engineering triiodothyronine (T3) nanoparticle for use in ischemic brain stroke. Drug Deliv Transl Res. 2013;3:309–17.
Wong Po Foo CTS, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc Natl Acad Sci. 2009;106:22067–72.
Franco CL, Price J, West JL. Development and optimization of a dual-photoinitiator, emulsion-based technique for rapid generation of cell-laden hydrogel microspheres. Acta Biomater. 2011;7:3267–76.
Baiguera S, Del Gaudio C, Lucatelli E, Kuevda E, Boieri M, Mazzanti B, et al. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials. 2014;35:1205–14.
Harris GM, Madigan NN, Lancaster KZ, Enquist LW, Windebank AJ, Schwartz J, et al. Nerve guidance by a decellularized fibroblast extracellular matrix. Matrix Biol. 2017;61:176–89.
Lee SJ, Zhu W, Nowicki M, Lee G, Heo DN, Kim J, et al. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J Neural Eng. 2017;15:1–29.
Zhu W, George JK, Sorger VJ, Zhang LG. 3D printing scaffold coupled with low level light therapy for neural tissue regeneration. Biofabrication. 2017;9:0–17.
Zhu W, Harris BT, Zhang LG. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2016;2016:4185–8.
Bhatia SK. Tissue engineering for clinical applications. Biotechnol J. 2010;5:1309–23.
El-Sherbiny I, Yacoub M. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract. 2013;2013:316–42.
Carballo-Molina OA, Velasco I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries. Front Cell Neurosci. 2015;9:1–12.
Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–63.
Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–26.
Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng B Rev. 2008;14:149–65.
Barros D, Amaral IF, Pêgo AP. Biomimetic synthetic self-assembled hydrogels for cell transplantation. Curr Top Med Chem. 2015;15:1209–26.
Nih LR, Carmichael ST, Segura T. Hydrogels for brain repair after stroke: an emerging treatment option. Curr Opin Biotechnol. 2016;40:155–63.
Skop NB, Calderon F, Cho CH, Gandhi CD, Levison SW. Improvements in biomaterial matrices for neural precursor cell transplantation. Mol Cell Ther. 2014;2:1–19.
Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–40.
Woerly S, Laroche G, Marchand R, Pato J, Subr V, Ulbrich K. Intracerebral implantation of hydrogel-coupled adhesion peptides tissue reaction. J Neural Transplant Plast. 1995;5:245–55.
Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34:5937–46.
Nomura H, Katayama Y, Shoichet MS, Tator CH. Complete spinal cord transection treated by implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery. 2006;59:183–92.
Pakulska MM, Ballios BG, Shoichet MS. Injectable hydrogels for central nervous system therapy. Biomed Mater. 2012;7:1–13.
Boisserand LSB, Kodama T, Papassin J, Auzely R, Moisan A, Rome C, et al. Biomaterial applications in cell-based therapy in experimental stroke. Stem Cells Int. 2016;2016:1–14.
James DT, Kyle JL. From de novo peptides to native proteins: advancements in biomaterial scaffolds for acute ischemic stroke repair. Biomed Mater. 2018;13:1–41.
Lutolf M. Designing materials to direct stem cell fate. Eur Cells Mater. 2011;462:433–41.
Lin X, Shi Y, Cao Y, Liu W. Recent progress in stem cell differentiation directed by material and mechanical cues. Biomed Mater. 2016;11:1–23.
Griffin MF. Control of stem cell fate by engineering their micro and nanoenvironment. World J Stem Cells. 2015;7:37–50.
Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A. Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int. 2015;2015:1–35.
Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–55.
Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. 2014;24:7053–62.
Lam J, Carmichael ST, Lowry WE, Segura T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv Healthc Mater. 2015;4:534–9.
Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, et al. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2:142–6.
Kuo Y-C. Chung C-Y. TATVHL peptide-grafted alginate/poly(γ-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials. 2012;33:8955–66.
Kyle JL, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9:5590–9.
Cheng T, Chen M, Chang W, Huang M, Wang T. Biomaterials neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2016;34:2005–16.
Wang X, Horii A, Zhang S. Designer functionalized self-assembling peptide nanofiber scaffolds for growth, migration, and tubulogenesis of human umbilical vein endothelial cells. Soft Matter. 2008;4:2388–95.
Liu X, Wang X, Horii A, Wang X, Qiao L, Zhang S, et al. In vivo studies on angiogenic activity of two designer self-assembling peptide scaffold hydrogels in the chicken embryo chorioallantoic membrane. Nanoscale. 2012;4:2720–7.
Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–38.
Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8.
Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–78.
Woerly S, Marchand R, Lavallée G. Intracerebral implantation of synthetic polymer/biopolymer matrix: a new perspective for brain repair. Biomaterials. 1990;11:97–107.
Murphy WL, Dennis RG, Kileny JL, Mooney DJ. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8:43–52.
Woerly S, Petrov P, Syková E, Roitbak T, Simonová Z, Harvey AR. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng. 1999;5:467–88.
Comolli N, Neuhuber B, Fischer I, Lowman A. In vitro analysis of PNIPAAm-PEG, a novel, injectable scaffold for spinal cord repair. Acta Biomater. 2009;5:1046–55.
Stabenfeldt SE, García AJ, LaPlaca MC. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J Biomed Mater Res A. 2006;77:718–25.
Tian WM, Zhang CL, Hou SP, Yu X, Cui FZ, Xu QY, et al. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: in vitro. J Control Release. 2005;102:13–22.
Kim DH, Seo YK, Thambi T, Moon GJ, Son JP, Li G, et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer. Biomaterials. 2015;61:115–25.
George PM, Bliss TM, Hua T, Lee A, Oh B, Levinson A, et al. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials. 2017;142:31–40.
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng A. 2009;15:3605–18.
Thrivikraman G, Madras G, Basu B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials. 2014;35:1–17.
Pires F, Ferreira Q, Rodrigues CAV, Morgado J, Ferreira FC. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta Gen Subj. 1850;2015:1158–68.
Luo Y, Shoichet MS. Light-activated immobilization of biomolecules to agarose hydrogels for controlled cellular response. Biomacromolecules. 2004;5:2315–23.
Goubko CA, Majumdar S, Basak A, Cao X. Hydrogel cell patterning incorporating photocaged RGDS peptides. Biomed Microdevices. 2010;12:555–68.
Emily RA, Kyle JL, Kimberly BB. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res. 2013;72:199–213.
Lin C-C, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–14.
Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31:553–6.
Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24:636–44.
Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355:1–18.
Ghuman H, Modo M. Biomaterial applications in neural therapy and repair. Chin Neurosurg J. 2016;2:1–8.
Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2010;93:1091–9.
Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–53.
Haile Y, Berski S, Dräger G, Nobre A, Stummeyer K, Gerardy-Schahn R, et al. The effect of modified polysialic acid based hydrogels on the adhesion and viability of primary neurons and glial cells. Biomaterials. 2008;29:1880–91.
Krsko P, McCann TE, Thach TT, Laabs TL, Geller HM. Libera MR. Length-scale mediated adhesion and directed growth of neural cells by surface-patterned poly(ethylene glycol) hydrogels. Biomaterials. 2009;30:721–9.
Arulmoli J, Wright HJ, Phan DTT, Sheth U, Que RA, Botten GA, et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016;43:122–38.
Graf J, Iwamoto Y, Sasaki M, Martin GR, Kleinman HK, Robey FA, et al. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987;48:989–96.
Bellamkonda RV, Ranieri JP, Aebischer P. Laminin oligopeptide derivatized agarose gels allow 3-dimensional neurite extension in vitro. J Neurosci Res. 1995;41:501–9.
Neiiendam JL, Køhler LB, Christensen C, Li S, Pedersen MV, Ditlevsen DK, et al. An NCAM-derived FGF-receptor agonist, the FGL-peptide, induces neurite outgrowth and neuronal survival in primary rat neurons. J Neurochem. 2004;91:920–35.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
Massensini AR, Ghuman H, Lindsey TS, Christopher JM, Timothy JK, Francesca JN, et al. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015;27:116–30.
Woerly S. Porous hydrogels for neural tissue engineering. Mater Sci Forum. 1997;250:53–68.
Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–92.
Hilfer R. Transport and relaxation phenomena in porous media. Adv Chem Phys. 1995;92:299–424.
Šprincl L, Kopeček J, Lím D. Effect of porosity of heterogeneous poly(glycol monomethacrylate) gels on the healing-in of test implants. J Biomed Mater Res. 1971;5:447–58.
Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology. 2016;68:355–69.
Whang K, Healy KE, Elenz DR, Nam EK, Tsai DC, Thomas CH, et al. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5:35–51.
Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517–24.
Klawitter JJ, Hulbert SF. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J Biomed Mater Res. 1971;5:161–229.
Gogolewski S, Pennings AJ. An artificial skin based on biodegradable mixtures of polylactides and polyurethanes for full-thickness skin wound covering. Macromol Rapid Commun. 1983;4:675–80.
Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci. 1989;86:933–7.
Schwartz I, Robinson BP, Hollinger JO, Szachowicz EH, Brekke J. Calvarial bone repair with porous D, L-polylactide. Otolaryngol Head Neck Surg. 1995;112:707–13.
Gerecht S, Townsend SA, Pressler H, Zhu H, Nijst CLE, Bruggeman JP, et al. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials. 2007;28:4826–35.
Lu L, Mikos AG. The importance of new processing techniques in tissue engineering. MRS Bull. 1996;21:28–32.
Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng B Rev. 2010;16:371–83.
Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, Eng G, et al. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–8.
Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–67.
Bae H, Nichol J, Foudeh A, Zamanian B, Kwon CH, Khadenhosseini A. Microengineering approach for directing embryonic stem cell differentiation. Stud Mechanobiol Tissue Eng Biomater. 2010;2:153–71.
Albert PJ, Schwarz US. Modeling cell shape and dynamics on micropatterns. Cell Adhes Migr. 2016;10:516–28.
Théry M. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci. 2010;123:4201–13.
Curley JL, Jennings SR, Moore MJ. Fabrication of micropatterned hydrogels for neural culture systems using dynamic mask projection photolithography. J Vis Exp. 2011;48:1–6.
Curley JL, Moore MJ. Facile micropatterning of dual hydrogel systems for 3D models of neurite outgrowth. J Biomed Mater Res A. 2011;99:532–43.
Rizwan M, Yahya R, Hassan A, Yar M, Azzahari AD, Selvanathan V, et al. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers. 2017;9:1–37.
Meng H, Hu J. A brief review of stimulus-active polymers responsive to thermal, light, magnetic, electric, and water/solvent stimuli. J Intell Mater Syst Struct. 2010;21:859–85.
Jeong B, Gutowska A. Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002;20:305–11.
Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications—a review. Eur J Pharm Biopharm. 2008;68:34–45.
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46.
Sá-Lima H, Tuzlakoglu K, Mano JF, Reis RL. Thermoresponsive poly(N-isopropylacrylamide)-g-methylcellulose hydrogel as a three-dimensional extracellular matrix for cartilage-engineered applications. J Biomed Mater Res A. 2011;98(A):596–603.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212.
Peterson DS. pH-sensitive hydrogel. Encycl Microfluid Nanofluidics. 2015:2726–9.
Kumar Dutta P, Dutta J, Tripathi VS. Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res. 2004;63:20–31.
Mukhopadhyay P, Sarkar K, Bhattacharya S, Bhattacharyya A, Mishra R, Kundu PP. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr Polym. 2014;112:627–37.
Atta S, Khaliq S, Islam A, Javeria I, Jamil T, Athar MM, et al. Injectable biopolymer based hydrogels for drug delivery applications. Int J Biol Macromol. 2015;80:240–5.
Wang C, Javadi A, Ghaffari M, Gong S. A pH-sensitive molecularly imprinted nanospheres/hydrogel composite as a coating for implantable biosensors. Biomaterials. 2010;31:4944–51.
Xiang Y, Liu HH, Bin YT, Zhuang ZQ, Jin DM, Peng Y. Functional electrical stimulation-facilitated proliferation and regeneration of neural precursor cells in the brains of rats with cerebral infarction. Neural Regen Res. 2014;9:243–51.
Brett Runge M, Mahrokh D, Jonas B, Terry R, Lichun L, Anthony JW, et al. Development of electrically conductive oligo (polyethylene glycol) fumarate-polypyrrole hydrogels for nerve regeneration. Biomacromolecules. 2010;11:2845–53.
Green RA, Hassarati RT, Goding JA, Baek S, Lovell NH, Martens PJ, et al. Conductive hydrogels: mechanically robust hybrids for use as biomaterials. Macromol Biosci. 2012;12:494–501.
Goding J, Gilmour A, Martens P, Poole-Warren L, Green R. Interpenetrating conducting hydrogel materials for neural interfacing electrodes. Adv Healthc Mater. 2017;6:1–13.
Ferraz N, Straømme M, Fellström B, Pradhan S, Nyholm L, Mihranyan A. In vitro and in vivo toxicity of rinsed and aged nanocellulose-polypyrrole composites. J Biomed Mater Res A. 2012;100 A:2128–38.
Humpolicek P, Kasparkova V, Saha P, Stejskal J. Biocompatibility of polyaniline. Synth Met. 2012;162:722–7.
Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29:3393–9.
Ter Schiphorst J, Coleman S, Stumpel JE, Ben Azouz A, Diamond D, Schenning APHJ. Molecular design of light-responsive hydrogels, for in situ generation of fast and reversible valves for microfluidic applications. Chem Mater. 2015;27:5925–31.
Tomatsu I, Peng K, Kros A. Photoresponsive hydrogels for biomedical applications. Adv Drug Deliv Rev. 2011;63:1257–66.
Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–60.
Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 2008;29:2962–8.
Tuladhar A, Morshead CM, Shoichet MS. Circumventing the blood-brain barrier: local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J Control Release. 2015;215:1–11.
Caicco MJ, Cooke MJ, Wang Y, Tuladhar A, Morshead CM, Shoichet MS. A hydrogel composite system for sustained epi-cortical delivery of cyclosporin A to the brain for treatment of stroke. J Control Release. 2013;166:197–202.
Wang Y, Cooke MJ, Morshead CM, Shoichet MS. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials. 2012;33:2681–92.
Wang Z, Wang J, Jin Y, Luo Z, Yang W, Xie H, et al. A neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke. ACS Appl Mater Interfaces. 2015;7:24629–40.
Shi W, Nie D, Jin G, Chen W, Xia L, Wu X, et al. BDNF blended chitosan scaffolds for human umbilical cord MSC transplants in traumatic brain injury therapy. Biomaterials. 2012;33:3119–26.
Shi W, Huang CJ, Xu XD, Jin GH, Huang RQ, Huang JF, et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016;45:247–61.
Addington CP, Heffernan JM, Millar-Haskell CS, Tucker EW, Sirianni RW, Stabenfeldt SE. Enhancing neural stem cell response to SDF-1α gradients through hyaluronic acid-laminin hydrogels. Biomaterials. 2015;72:11–9.
Betancur MI, Mason HD, Alvarado-Velez M, Holmes PV, Bellamkonda RV, Karumbaiah L. Chondroitin sulfate glycosaminoglycan matrices promote neural stem cell maintenance and neuroprotection post-traumatic brain injury. ACS Biomater Sci Eng. 2017;3:420–30.
Führmann T, Obermeyer J, Tator CH, Shoichet MS. Click-crosslinked injectable hyaluronic acid hydrogel is safe and biocompatible in the intrathecal space for ultimate use in regenerative strategies of the injured spinal cord. Methods. 2015;84:60–9.
Caron I, Rossi F, Papa S, Aloe R, Sculco M, Mauri E, et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135–47.
Zhao YZ, Jiang X, Xiao J, Lin Q, Yu WZ, Tian FR, et al. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater. 2016;29:71–80.
Caicco MJ, Zahir T, Mothe AJ, Ballios BG, Kihm AJ, Tator CH, et al. Characterization of hyaluronan-methylcellulose hydrogels for cell delivery to the injured spinal cord. J Biomed Mater Res A. 2013;101:1472–7.
Lonardo E, Parish CL, Ponticelli S, Marasco D, Ribeiro D, Ruvo M, et al. A small synthetic cripto blocking peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of Parkinson’s disease. Stem Cells. 2010;28:1326–37.
Nakaji-Hirabayashi T, Kato K, Iwata H. Hyaluronic acid hydrogel loaded with genetically-engineered brain-derived neurotrophic factor as a neural cell carrier. Biomaterials. 2009;30:4581–9.
Li J, Darabi M, Gu J, Shi J, Xue J, Huang L, et al. A drug delivery hydrogel system based on activin B for Parkinson’s disease. Biomaterials. 2016;102:72–86.
Komatsu M, Konagaya S, Egawa EY, Iwata H. Maturation of human iPS cells-derived dopamine neuron precursors in alginate-Ca(2+) hydrogel. Biochim Biophys Acta. 1850;2015:1669–75.
Bastiancich C, Vanvarenberg K, Ucakar B, Pitorre M, Bastiat G, Lagarce F, et al. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Control Release. 2016;225:283–93.
Jin H, Zhao G, Hu J, Ren Q, Yang K, Wan C, et al. Melittin containing hybrid peptide hydrogels for enhanced photothermal therapy of glioblastoma. ACS Appl Mater Interfaces. 2017;9:25755–66.
Fourniols T, Randolph LD, Staub A, Vanvarenberg K, Leprince JG, Préat V, et al. Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J Control Release. 2015;210:95–104.
Tsao CT, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, et al. Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel. Biomacromolecules. 2014;15:2656–62.
Katz JS, Burdick JA. Hydrogel mediated delivery of trophic factors for neural repair. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:128–39.
Wang Y, Cooke MJ, Sachewsky N, Morshead CM, Shoichet MS. Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J Control Release. 2013;172:1–11.
Schäbitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances post stroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–72.
Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–6.
Ren JM, Finklestein SP. Growth factor treatment of stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:121–5.
Cook DJ, Nguyen C, Chun HN, L Llorente I, Chiu AS, Machnicki M, et al. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. 2017;37:1030–45.
Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38.
Emerich DF, Silva E, Ali O, Mooney D, Bell W, Yu SJ, et al. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010;19:1063–71.
Yamashita T, Deguchi K, Nagotani S, Abe K. Vascular protection and restorative therapy in ischemic stroke. Cell Transplant. 2011;20:95–7.
Ju R, Wen Y, Gou R, Wang Y, Xu Q. The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant. 2014;23:1–38.
Bath PMW, Lees KR. Acute stroke. West J Med. 2000;173:209–12.
Bernstock JD, Peruzzotti-Jametti L, Ye D, Gessler FA, Maric D, Vicario N, et al. Neural stem cell transplantation in ischemic stroke: a role for preconditioning and cellular engineering. J Cereb Blood Flow Metab. 2017;37:2314–9.
Wechsler LR. Stem cell therapies as an emerging paradigm in stroke (STEPS) bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–5.
Wei Z, Zhao J, Chen YM, Zhang P, Zhang Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci Rep. 2016;6:1–12.
Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed post ischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–44.
Fujioka T, Kaneko N, Ajioka I, Nakaguchi K, Omata T, Ohba H, et al. β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine. 2017;16:195–203.
McMurtrey RJ. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J Neural Eng. 2014;11:1–15.
Navaei-Nigjeh M, Amoabedini G, Noroozi A, Azami M, Asmani MN, Ebrahimi-Barough S, et al. Enhancing neuronal growth from human endometrial stem cells derived neuron-like cells in three-dimensional fibrin gel for nerve tissue engineering. J Biomed Mater Res A. 2014;102:2533–43.
Chen SJ, Chang CM, Tsai SK, Chang YL, Chou SJ, Huang SS, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19:1757–67.
Ma T, Wang Y, Qi F, Zhu S, Huang L, Liu Z, et al. The effect of synthetic oxygen carrier-enriched fibrin hydrogel on Schwann cells under hypoxia condition in vitro. Biomaterials. 2013;34:10016–27.
Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A. 2014;111:13805–10.
Mosley MC, Lim HJ, Chen J, Yang YH, Li S, Liu Y, et al. Neurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young’s modulus gradient. J Biomed Mater Res A. 2017;105:824–33.
Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–93.
Jendelová P, Kubinová Š, Sandvig I, Erceg S, Sandvig A, Syková E. Current developments in cell- and biomaterial-based approaches for stroke repair. Expert Opin Biol Ther. 2016;16:43–56.
Ghumana H, Massensini AR, Julia D, Kim SM, Christopher JM, Stephen FB, et al. ECM hydrogel for the treatment of stroke: characterization of the host cell infiltrate. Biomaterials. 2016;14:166–81.
Jin T, Nicholls FJ, Crum WR, Ghuman H, Badylak SF, Modo M. Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke. Biomaterials. 2017;113:176–90.
Boisserand LSB, Lemasson B, Hirschler L, Moisan A, Hubert V, Barbier EL, et al. Multiparametric magnetic resonance imaging including oxygenation mapping of experimental ischaemic stroke. J Cereb Blood Flow Metab. 2017;37:2196–207.
Liang Y, Walczak P, Bulte JWM. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials. 2013;34:5521–9.
Lugo-Hernandez E, Squire A, Hagemann N, Brenzel A, Sardari M, Schlechter J, et al. 3D visualization and quantification of microvessels in the whole ischemic mouse brain using solvent-based clearing and light sheet microscopy. J Cereb Blood Flow Metab. 2017;37:3355–67.
Raza F, Zafar H, Zhu Y, Ren Y, Ullah A, Khan AU, et al. A review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable polymers. Pharmaceutics. 2018;10:1–21.
Tronci G, Ajiro H, Russell SJ, Wood DJ, Akashi M. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014;10:821–30.
Webber MJ, Khan OF, Sydlik SA, Tang BC, Avenue MA. Perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng. 2016;43:641–56.
Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 2015;370:1–8.
Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gopalakrishnan, A., Shankarappa, S.A. & Rajanikant, G.K. Hydrogel Scaffolds: Towards Restitution of Ischemic Stroke-Injured Brain. Transl. Stroke Res. 10, 1–18 (2019). https://doi.org/10.1007/s12975-018-0655-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0655-6