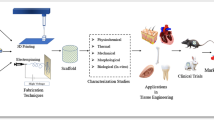
Abstract
Tissue engineering is approach of replacing or regeneration of biological functions of tissues or organs by using combination of biomaterials, biomolecules and cells. Tissue engineering mainly depends scaffold biomaterials and scaffold fabrication methods. Therefore, there have been progressive investigation and development of new biomaterials with different formulations to help and achieve necessary requirements in the tissue engineering applications. This review is briefly representing necessary features associated with biomaterial type and design required for tissue regeneration applications, and presenting earlier research in tissue engineering field and new trends for future implementation. It is mainly focusing on generations of biomaterials and discovery tissue engineering field. As well as, different types of biomaterials, such as bioceramics, bioactive glasses, synthetic and natural polymers and their derived composites, used in fabrication of scaffolds (as a main part of tissue engineering) are demonstrated in this review. Scaffold fabrication methods are also reviewed here. Moreover, it is showing the recent achievements in tissue engineering field for bone, skin, cartilage, neural, and cardiac regeneration as a pre-clinical procedure for repair of injured and diseased tissues and organs. Finally, recent trends and challenges of biomaterials for tissue regeneration are presented also in this review.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biomaterials and tissue engineering
Definition of biomaterials employed by the National Institute of Health (NIH) defines biomaterial as “any natural or synthetic substance or combination of substances, other than drugs, which can be used to augment or partially or totally replace any tissue, organ or function of the body, in order to maintain or improve quality of life of individual”. Biomaterials have gone through several stages of evolution which could be divided into three generations. First generation biomaterials are known as bioinert, where materials could not react with the host tissue at the interface between both of them. Second generation biomaterials are known as bioactive materials, where the material could form interfacial bonding with tissue. Third generation biomaterials are being designed to stimulate specific cellular responses at the molecular level using bioactive and bioresorbable in the form of interconnected porous architecture. Figure 1 represents different generations of biomaterials.
Generations of biomaterials; 1st generation biomaterial (bioinert material), forms fibrous tissue around it, 2nd generation biomaterial (bioactive material), forms chemical bond at the interface between its surface and surrounding tissue, 3rd generation biomaterial (regenerative material), enhances tissue healing and degrades thereafter
Tissue regeneration and tissue engineering have become synonymous terms in the field of diseased tissue and organ treatments. Tissue engineering is a rising biomedical field intended to repair and restore tissue defects by a combination of biomaterials living cells [1,2,3]. Tissue engineering is a promising technique for tissue regeneration in situ by incorporating cells into bioactive scaffolds. It has emerged as an alternative method for grafting and transplantation of diseased or damaged tissue. Recently, tissue engineering developed progressively as a result of designing of novel materials to restore tissue function. Nevertheless, scaffolds are playing an important role throughout tissue regeneration process. Their 3D porous structure designed to provide structural support for cell attachment and migration through pore channels and subsequent tissue development. Figure 2 represents steps of culturing cells onto scaffold and how the tissue is formed ultimately. The ideal scaffold should possess the following characteristics to bring about desired biologic response [4];
-
1.
3D interconnected porous network to facilitate cell to get rid of metabolic waste and nutrients flow.
-
2.
Possesses controllable degradation and resorption rate to match the cell growth.
-
3.
Suitable surface roughness and chemistry for cell attachment.
-
4.
Mechanical properties to match those of tissues at the site of implantation.
-
5.
Can be prepared in a variety of shapes and sizes.
-
6.
It should simulate the extracellular matrix (ECM) in both biological function and structure.
Schematic diagram showing steps of cell culture on scaffold for tissue engineering purpose. This figure is reproduced from [5] under an open access license distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license Copyright
Due to biomaterials potential in tissue regeneration applications, the number of published articles of biomaterials tissue engineering is continuously increased every year, where about 12,518 articles were published during 20 years. Figure 3 represents the number of publications corresponding to the specialization field.
Types of biomaterials used for scaffolds
Bioceramics
Ceramics are inorganic non-metallic materials; they are one of the oldest materials known by humankind. They have widely been utilized in optical, electronic, energy-related and biomedical applications. In the last decades, bioceramics (e.g., hydroxyapatite, zirconia, alumina, tricalcium phosphates and bioactive glasses) gained a special interest in the biomedical applications, such as restoring and substituting hard tissues like bone, teeth, hip joints and bone. Meanwhile, bioceramics reported to present better tissue responses than metals and polymers. That is because their good biocompatibility with cells, as well as, some types of bioceramics, such as hydroxyapatite and bioactive glass, can form bond directly with bone.
Generally, bioceramics are classified according to their ability to bind with the bone into two large families: bioinert (such as alumina and zirconia) and bioactive ceramics (such as hydroxyapatite, bioactive glasses and bioactive glass–ceramics). Since we are talking here about biomaterials that have ability to regenerate tissues, we will focus on bioactive materials only.
Bioactive glass and bioactive glass–ceramics
Bioactive glasses are the most interesting bioceramic materials for bone defects and soft tissue treatments during the last decades and they belong to second and third generations of biomaterials. That is because of their unique ability to convert to hydroxyapatite (HA) in vivo, and their ability to bond with bone and soft tissues [6,7,8]. The first bioactive glass, which was discovered by Hench et al. [6] in the late 1960s and early 1970s, was prepared by conventional melting method. It encoded later as 45S5 or Bioglass® and it opened a new research field by using glasses as implants and bone tissue engineering applications. The composition of such glass was based on 45.0 SiO2—24.5 CaO—24.5 Na2O—6.0 P2O5 glass system in wt%. This glass proved to form chemical bonds with host tissue through formation of new hydroxyapatite layer.
Mechanism of formation of new hydroxyapatite layer at the interface of glass with surrounding tissue has been described extensively by Hench [6, 9, 10] and others [11, 12]. When glass surface is subjected to body fluid, hydroxyapatite layer is formed according to the following stages:
-
Stage 1 An exchange of Na+ in glass with H+ or H3O+ in solution takes place, which occurs rapidly during initial minutes after exposure of glass surface body fluid.
$${\text{Si}} - {\text{O}} - {\text{Na}}^{ + } + {\text{ H}}^{ + } + {\text{ OH}}^{ - } \to {\text{ Si}} - {\text{OH }} + {\text{ Na}}^{ + }_{{({\text{solution}})}} + {\text{ OH}}^{ - }$$ -
Stage 2 Loss of soluble silica as Si(OH)4by breaking of Si–O–Si bridges due to loss of Na+and subsequent formation of surface silanol (Si–OH) groups in process.
$${2}\left( {{\text{Si}} - {\text{O}} - {\text{Si}}} \right) + { 2}\left( {{\text{OH}}} \right) \, \to {\text{ Si}} - {\text{OH }} + {\text{ HO}} - {\text{Si}}$$ -
Stage 3 Condensation and repolymerization of surface silanols to form an SiO2-rich surface layer.
$${2}\left( {{\text{Si}} - {\text{OH}}} \right) \, + { 2}\left( {{\text{HO}} - {\text{Si}}} \right) \, \to \, - {\text{Si}} - {\text{O}} - {\text{Si}} - {\text{O}} - {\text{Si}} - {\text{O}} - {\text{Si}} - {\text{O}} -$$ -
Stage 4 Migration of Ca2+ and PO43− to the surface through silica-rich layer and formation of an amorphous Ca–P-rich layer on surface of glass by incorporation of soluble calcium and phosphate from solution.
-
Stage 5 Incorporation of OH−, CO32− from the solution and subsequent crystallization of Ca–P layer to form hydroxyl carbonated apatite (HCA).
Figure 4 represents surface stages reaction on bioactive glass forming HA layer which forms a chemical bond with glass surface and surrounding tissue.
There have been several bioactive glasses developed thereafter discovering of Hench glass other than silicate-based glass, such as borate- and phosphate-based compositions. Recently, borate glass has used interestingly as a bioactive material due to its lower chemical durability and faster completely conversion to an HA phase than other well-known 45S5 glass [13, 14]. Moreover, borate bioactive glasses have proved to induce cell differentiation and proliferation in vitro, as well as tissue regeneration in vivo [15]. Phosphate glasses, mainly based on Na2O–CaO–P2O5 system, have been applied in biomedical applications. Because of their similar chemical composition to an inorganic part of bone, phosphate glassed have been successfully used as resorbable materials for bone therapy [8]. Such type of glass is better than hydroxyapatite ceramic, because of a possibility to control its solubility by modifying the composition by incorporating other modifying oxides [16].
In the past decades, bioactive glasses have been prepared via conventional melting method, i.e., by melting mixed oxides of glass constituents at high temperature and quenching the melt in air [17]. Although this method is cheap and simple, it still has some shortages, such as inhomogeneity and difficulty to obtain nanoscale particles. However, glass bioactivity is directly related to the rate of dissolution of glass and its morphology. Accordingly, as the specific surface area of glass increases, the dissolution rate increases, and hence, the bioactivity increases. Therefore, preparation of glass particles in the nanoscale has been urgently required. As a result, there have been different methods developed to prepare nanobioactive glass (NBG) (1–100 nm particle size), such as sol–gel techniques, microemulsion techniques, gas phase synthesis method (flame spray synthesis) and laser spinning techniques. This review focused mainly on the sol–gel process.
In 1991, the incorporation of sol–gel chemistry gave rise to a new generation of bioactive glasses with a great potential to develop better implants for biomedical applications [18, 19]. This fact was attributed to the enhanced surface area, porosity and much lower glass synthesis temperature derived from the sol–gel process, in comparison with melting and quenching techniques used for the synthesis of conventional glasses [20]. Moreover, the advances in novel synthesis processes have allowed researchers to obtain nanomaterials based on sol–gel bioactive glass as nanoparticles and nanofibers, which are promising candidates for biomedical applications.
Process of sol–gel is a polymerization of oxide liquid precursors from solution via a conversion from liquid to a “sol” and finally to a network structure called a “gel” [21]. Generally, sol occurs by hydrolysis and condensation of metal alkoxide (mainly, silicon alkoxide) precursors, and they are affected by several factors, such as nature of alkyl group (R-group), ratio of water to alkoxide and including of catalysts (mainly acid or base catalyst) in the reaction.
In the hydrolysis step, an alkoxy group in silicon alkoxide (it is a well-known example for metal alkoxide used in synthesis of nanobioactive glass by sol–gel method) is replaced by a hydroxyl group and forms a transition state compound with penta-coordinate geometry in both acid and base (Fig. 5) catalyzed hydrolysis. Therefore, Si:H2O ratio directly affects on the rate of hydrolysis. According to the following equation, each 1 mol of alkoxide needs 4 mol of H2O to be completely hydrolyzed. Therefore, the molar ratio between H2O and Si(OR)4 is 4:1, and the rate of hydrolysis becomes faster if this ratio is increased, while it gets slower when it is decreased [22]. On the other hand, progressive hydrolysis of alkoxides is getting slower at lower pH and faster at higher one.
Hydrolysis mechanism of silicon alkoxide. a is a mechanism of acid catalyzed condensation of silicon alkoxide. b is a mechanism of base catalyzed condensation of silicon alkoxide. Adapted from [21], Copyright
In the condensation step, all hydrolyzed alkoxide starts to condense by losing H2O molecules and two Si atom attached with each other via bridging oxygen, namely known as “siloxane bond”. The mechanism of condensation is the same for acid and base catalyzed reactions as shown in Fig. 5. In fact, condensation progress of Si alkoxides depends on the hydrolysis degree in hydrolysis step of such alkoxides to transform to silanol (Si–OH) groups. Previously completed hydrolysis gives (OH)3Si–O–Si(OH)3 species which has 6 sites for the ongoing condensation reaction. Figure 6 represents progress of condensation of silanol groups and formation of 3D interconnected glass network. As a result of continuous condensation steps, highly branched and agglomerates with small size formed in the solution are crosslinked to form gel.
Condensation of silanol (Si–OH) groups and formation of 3D interconnected glass network. Adapted from [21], Copyright
Glass–ceramic is a polycrystalline material obtained by controlled crystallization of glass via specific heat treatment of the parent glass [23]. Bioactive glass–ceramics have been utilized for more than three decades in biomedical applications, and in last years, they have been used in bone tissue engineering applications. The first macroporous scaffolds based on bioactive glass were prepared in the early 2000s using foaming techniques to Bioglass® by H2O2 foaming solution [24]. Since this time, a lot of researches have been performed to fabricate bioactive glass-based scaffolds in numerous shapes and sizes to reach to the ideal scaffold for bone regeneration. The main critical issue in this case was producing scaffolds with mechanical properties that best match those of human bone [25]. There is a reverse relation between porosity and mechanical properties in the scaffold, as the porosity increases the mechanical strength decreases. But, numerous research works were able to fabricate glass scaffolds with desirable mechanical properties for the bone, as shown in Fig. 7. Fu et al. [26] prepared bioactive glass (13–93) scaffolds with lamellar oriented porous structure of porosity 55–60%, pore width of 90–110 µm, compressive strength 25 ± 3 MPa and compressive modulus of 1.2 GPa. This compressive strength value of the prepared scaffolds was higher than strength of trabecular bone by > 1.5 times. They also synthesized bioactive glass–ceramic scaffolds substituted with magnesium and potassium with porosity of 85 ± 2%, pore size of 100–500 μm and compressive strength of 11 ± 1 MPa and compressive modulus of 3.0 ± 0.5 GPa, which corresponded to the human trabecular bone [27] (compressive strength of trabecular bone is 2–12 MPa [28, 29]). Baino et al. [30] fabricated fluoroapatite glass–ceramic scaffolds. The resulted scaffolds were characterized by porosity ranged from 23.5 to 50%, and compression strength of 20–150 MPa. Farag et al. [31] fabricated glass–ceramic scaffolds by replica method based on different ratios between Bioglass® and glass with low crystallization affinity acted as a glass matrix for crystallized glass particles. Degradation of the resulted scaffolds could be tailored by changing the percentages of both glasses.
Porosity against compressive strength of different glass scaffolds compared with the human bone. Reproduced with permission from [25], Copyright
Bioactive glass scaffolds have been progressively developed, and they fabricated not only to regenerate the bone tissue, but also they possessed more therapeutic functions, such as cancer and osteomyelitis treatments. Chengtie et al. [32] prepared multifunctional magnetic mesoporous bioactive glass scaffolds by replica method, and they studied the effect of iron on the mesopore structure, drug delivery, magnetic and biological properties. The resultant scaffolds considered a good candidate to treat and regenerate bone defects, as well as, they demonstrated a combined ability of local drug delivery, hyperthermia and osteoconductivity, and so, they were suitable for bone cancer treatment and bone regeneration. The same authors fabricated multifunctional bioactive glass scaffolds contained copper characterized by osteostimulation, angiogenesis capacity, as well as, they were loaded with ibuprofen drug as antibacterial drug.
In the last years, bioactive glass scaffolds have been colonized with live cells to integrate and stimulate growth of tissue. Clemens et al. [33] were prepared highly porous novel bioactive glass scaffolds seeded with undifferentiated human mesenchymal stromal cells and porcine articular chondrocytes for cartilage tissue engineering. The scaffolds showed high cell biocompatibility, and type II collagen protein expression and cartilage-specific proteoglycans of articular chondrocytes revealed the preservation of their chondrogenic phenotype.
Calcium phosphates
Calcium phosphates are a major family of bioceramic materials. They have been widely used in dentistry, drug delivery and bone regeneration, because they exhibit good biocompatibility with the surrounding tissues when implanted in the body. Moreover, hydroxyapatite, one type of Ca-phosphates, is the main constituent of inorganic part of natural bone which forms 60% of bone. And so, in the past decades, calcium phosphates have been used as bone substitutes. Table 1 presents different types of Ca-phosphate depending on Ca/P ratio. It was reported that Ca-phosphates of Ca/P ratio < 1 are not appropriate for biological implantation due to high dissolution rate of those Ca-phosphates. But, resorption rate of Ca-phosphates with Ca/P > 1.67 dramatically decreases. [34].
Hydroxyapatite (Ca10(PO4)6(OH)2, HAp) and tricalcium phosphate (Ca3(PO4)2, TCP) have been drawn the attention due to their outstanding osteoconduction and biocompatibility. As a result, these biomaterials have been successfully used as bulk or bioactive cement constituent materials in tissue regeneration in the last years. Accordingly, this review is focusing on application of such materials in the tissue engineering. Wei et al. [36] were fabricated scaffolds based on HAp and magnesium ammonium phosphate hexahydrate (NH4MgPO4·6H2O) with different ratios and hierarchical pore sizes for bone tissue regeneration. The scaffold porosities and pore size were in the range 52–78% and 400–500 µm, respectively. Moreover, scaffolds showed good cell attachment and proliferation specifically for Mg–Ca-phosphate scaffolds, and they enhanced new bone regeneration in the in vivo test. Ca-phosphate scaffolds were loaded with rhBMP-2 (human bone morphogenetic protein-2) by Zhang et al. [37] to enhance bone tissue regeneration. They in vitro examined these scaffolds with C2C12 model cells and in vivo tested in rabbit femur defect to evaluate osteogeneic function of the combined scaffolds. The results demonstrated that scaffolds combined with rhBMP-2 were intensely stimulated osteogenic differentiation with C2Cl2 cells and in vivo promoted osteogenetic efficacy.
Magnesium phosphates
Magnesium phosphate ceramics (MPCs) have been possessed an increasing interest in the last decades for dentistry and bone regeneration, because they introduce solutions to some limitations of Ca-phosphate ceramics, such as biodegradability and mechanical strength. As well as, derived Mg-phosphate cements have been potentially used in orthopedics. The biocements are mainly based on struvite crystals (magnesium ammonium phosphate hexahydrate, MgNH4PO4·6H2O) formation [38,39,40]. Such cements are illustrated high mechanical strength relative to other bone substitutes, like brushite, as well as, they have biodegradability analogous to brushite [41]. On the other hand, they showed good biocompatibility with the bone cells [42, 43]. Also, they demonstrated activity against several antibacterial species [44]. Moreover, Mg is fourth most abundant cation in the human body [45], and secondly rich cation, after potassium, in the intracellular matrix [46], it is naturally located in bone, too [47]. Furthermore, Mg was reported to decrease bone fragility [48].
According to these advantages, MPCs and their derived cements assigned to be used as good candidates in bone tissue regeneration. Our research group fabricated Mg-phosphate scaffolds loaded with lysozyme drug by paste extruding deposition rapid prototyping technique for hard tissue regeneration and antibacterial applications. The obtained scaffolds characterized by well-interconnected porous structures, desirable mechanical strength, biodegradability and auspicious cell viability [49]. In a continued work, we fabricated hybrid Mg-phosphate and gelatin polymer scaffold with different ratios by additive manufacturing technique. The results of this study showed that addition of gelatin was greatly improved mechanical strength of the scaffold (compressive strength was16.7 ± 1.9 MPa), cell viability and drug release profiles [39]. Götz et al. [50] fabricated calcium magnesium phosphate scaffolds and studied effect of powder/liquid ratio and hydroxypropylmethylcellulose (HPMC) on ceramic paste rheological properties. The scaffolds were showed compressive strength in the range 1.6–3.0 MPa. Farag et al. [51] added magnesium phosphate to nanobioactive glass (NBG), based on 85SiO2-10CaO-5P2O5 (mole %), with different ratios using the sol–gel route to tailor the biodegradation and biocompatibility of the final composites. The results showed that addition of Mg-phosphate was increased the degradation of NBG, and Mg-phosphate enhanced the cell viability of NBG in the ceramic composites. Farag et al. [52] prepared Mg-phosphate/bacterial cellulose and struvite/bacterial cellulose composites by green method using bacterial strain Gluconacetobacter xylinum ATCC 10,245, and studied the effect of Mg-phosphate-based material type on the green synthesis process, biodegradation and biocompatibility. The distinctive results showed that both composites enhanced formation of bone-like apatite layer on their surfaces after immersion in SBF, and this was dominant in case of struvite/bacterial cellulose composite.
Polymeric biomaterials
Polymers are the most commonly used materials in tissue regeneration, specially, for cardiovascular, skin and other soft tissues. Biodegradable polymers are subdivided according to its origin into two groups, natural polymers and synthetic polymers. Figure 8 represents structure of different biodegradable natural polymers (collagen, gelatin, alginate and chitosan) and synthetic polymers (poly(l-lactic acid) (PLLA), poly(l-lactic-co-glycolic acid) and poly(ε-caprolacton) (PCL)). The following section reviewed previous studies applied biodegradable polymers in tissue regeneration application.
Natural polymeric biomaterials
Collagen
Collagen is a high molecular weight polymer composed of amino acid monomers. It is the most abundant protein in the human body and is mainly found in cartilage, ligament, skin, tendon and is the main component of organic part of bone. It is composed of strands of polypeptides containing tri-amino acid blocks of glycine and its other amino acids derivatives, commonly proline and hydroxyproline [53].
Gelatin
Gelatin is produced from thermal denaturation by controlled hydrolysis of collagen extracted from tissues of animals, such as skin and bovine and porcine bone [54]. Gelatin is used potentially as drug delivery formulations in the shape of capsules for oral administration or microspheres for local delivery systems. Moreover, gelatin has been used successfully in soft and hard tissue engineering applications, and its impact increased when it was mixed with bioactive glass nanoparticles.
Alginate
Alginate is widely used in different biomedical applications, such as wound healing, tissue engineering, injectable bone cements and drug delivery applications. That was because of its low toxicity, excellent biocompatibility, its effective cost and its great similarity in structure to the extracellular matrices in tissues. Alginate is a natural polymer likely extracted from brown algae. Alginate salt (usually, alginate sodium salt) is composed of β-d-mannuronic acid (known as M blocks) and its C-5 epimer α-l-guluronic acid (known as G blocks) residues.
Chitosan
Chitosan is a natural polymer derived industrially from chitin of crustaceans and fungal mycelia. It is a semi-crystalline polysaccharide polymer. It is composed of N-acetyl d-glucosamine and d-glucosamine units (Fig. 8) [55]. Due to the unique properties, such as biodegradability, mucoadhesion and hemostatic activity, its antibacterial and antifungal activities and cell compatibility make this polymer widely used in different biomedical applications. Chitosan is a well-known polymer for synthesis of biomaterials, such as hydrogels, tissue engineering scaffolds, injectable bone implant materials and wound dressing. Despite all advantages of chitosan, it still lacks bioactivity feature like other biodegradable polymers. In this respect, different bioactive materials, such as hydroxyapatite and bioactive glass, could be introduced to enhance and improve bioactivity of chitosan. Bioactive glass nanoparticles are excellent candidates as bioactive filler for chitosan due to their high surface area, osteoconductivity and osteoinductivity.
Synthetic polymeric biomaterials
Poly(lactic acid) (PLA)
Due to its biodegradability, biocompatibility and good mechanical properties, poly(lactic acid) (PLA) is widely used in different biomedical fields, such as bone fixation, injectable microspheres, drug delivery system and tissue engineering applications. PLA is thermoplastic aliphatic polyester. It is mainly prepared from lactic acid which exists as two enantiomers, l- and d-lactic acid or mixtures of both components. As a result, there are poly(d-lactide acid) (PDLA) and poly(l-lactide acid) (PLLA). PLLA polymer is characterized by its excellent biocompatibility and mechanical strengths, and it has been approved by the Food and Drug Administration (FDA). Therefore, most of researchers have been used PLLA for different biomedical applications.
Poly(lactic-co-glycolic acid) (PLGA)
Like poly(lactic acid), poly(lactic-co-glycolic acid) (PLGA) polymer has potential application in the biomedical field, and it also has been approved by the Food and Drug Administration (FDA). It is obtained by copolymerization of lactic acid and glycolic acid. In comparison with PLA, PLGA polymer is a more hydrophilic degradable polymer than PLA. However, degradation of PLGA can be controlled by changing of the ratio of lactide to glycolide monomers.
Poly(ε-caprolacton) (PCL)
Poly(ε-caprolacton) (PCL) is a biodegradable polyester, and it is prepared by the ring opening polymerization of ε-caprolactone. It also has been approved by the Food and Drug Administration (FDA) for the use in humans, such as bone filling, drug delivery devices, suture and tissue engineering. Compared with previously mentioned PLA and PLGA polymers, PCL is cheaper, has better processibility and it has high thermal stability which enables it to be shaped by melting process.
Composites
Composite biomaterials are materials that made from two or more types of materials. Composite biomaterials combine between the advantages of each composite-made phase. For example, the composites based on bioactive glasses and biodegradable polymers mix the benefits of both phases; bioactivity property, which comes from bioactive glass particles and gains flexibility characteristic which comes from polymer. Such new material family has opened the field to be applied in more versatile tissue regeneration. Therefore, the number of published articles of composite biomaterials for tissue regeneration is progressively increased every year.
Kim et al. [56] prepared scaffold nanofibers based on 58S nanobioactive glass and collagen by electrospinning technique. The composites represented favorable growth of osteoblastic cells during in vitro cell test. Bae et al. [57] developed a new bioactive glass/collagen nanocomposite scaffolds for dentin-pulp regeneration. They studied influence of such materials on proliferation and differentiation of human dental pulp cells (hDPCs). It was reported in their study that incorporation of bioactive glass nanoparticles improved formation of hydroxyapatite crystals during in vitro study in simulated body fluid (SBF). Furthermore, the nanocomposite more significantly induced growth and proliferation of hDPCs than collagen-based scaffold. Marelli et al. [58] introduced bioactive glass nanoparticles into collagen polymer to improve bioactivity of nanocomposite scaffold for bone tissue engineering application. Beside its role to accelerate apatite bone-like minerals, glass nanoparticles also improved the compressive modulus of the scaffold. Farag et al., [39] fabricated composite Mg-phosphate/gelatin scaffolds by additive manufacturing technique. The results of this study showed that addition of gelatin was greatly improved the mechanical strength of the scaffold (compressive strength was 16.7 ± 1.9 MPa), cell viability and drug release profiles. Mozafari et al. [59, 60] prepared bioactive glass/gelatin nanocomposite scaffolds by solvent casting combined with freeze-drying and lamination techniques. The resulted scaffold was characterized by interconnected macroporous structure with pore sizes ranged from 200 to 500 μm. Inclusion of bioactive glass nanoparticles into gelatin polymer improved formation of bone-like apatite mineral on the surface of scaffold, as well as, it enhanced cell attachment on its surface. Lei et al. [61] fabricated high compressive strength silicate bioactive glass/gelatin bone implant. The implant showed compressive strength of about 120 MPa, as well as, apatite-like layer was uniformly formed on its surface when it was immersed in SBF after a short time. Furthermore, it enhanced marrow stem cells growth and proliferation when they were in vitro cultured on the implant surface. Therefore, the prepared nanocomposite implant was proposed to be used for bone fixation and repair biomaterials. Zhihua et al. [62] developed nanocomposite scaffolds for tissue engineering applications based on nanobioactive glass and a blend of gelatin and hyaluronic acid. Bioactive glass nanoparticles obviously enhanced compressive strength, formation apatite-like layer and cell viability of the final composite scaffold. Gönen et al. [63] prepared nanocomposite scaffolds from nonwoven fiber based on bioactive glass nanoparticles and a mixture of gelatin and poly(ε-caprolactone) using the electrospinning technique. The diameter of building fiber was around 584 nm and final scaffold showed an interconnected macroporous structure as it was expected, glass particles improved bioactivity and cell viability of the scaffold. Sharifi et al. [64] fabricated scaffolds from copper containing bioactive glass fibers and a polymer blend of gelatin and collagen. They enhanced mechanical strength of the scaffold by using genipin cross-linker. On the other hand, copper containing glass composite scaffold enhanced cell attachment and growth more than copper-free glass composite scaffold. Yu et al. [65] functionalized nano-hydroxyapatite/silk fibroin composite scaffolds with naringin to enhance healing of vertebral defects in ovariectomised rat. The fabricated scaffolds revealed good biocompatibility, cell viability and biomechanical strength. As well as, naringin improved in vitro osteogenic differentiation. The scaffolds regenerate bone defects which recover in 16 weeks. Their results recommended the prepared scaffolds for bone regeneration. Wang et al. [66] incorporated vascular endothelial growth factor (VEGF) in hydroxyapatite/collagen scaffolds. The scaffolds exhibited good biocompatible composite scaffolds with excellent angiogenic properties.
In addition, inorganic nanocomposites based on bioceramics and metal oxides nanoparticles have drawn a special interest. Bhushan et al. [67] synthesized magnetic and antibacterial bioceramic nanocomposites based on α-Fe2O3 and MnO wet-chemical method. Their results showed that the prepared composites demonstrated superparamagnetic properties at room temperature and excellent antibacterial activity against different bacteria strains. Montazeran et al. [68] added magnetite to calcium silicate to reinforce its mechanical strength and magnetic properties and applied the artificial neural network to the previous data to estimate the biological and mechanical properties of those composites as output parameters and compare them with present experimental data. The results presented that the modeling products were close to experimental values.
The composites based on HAp and zirconia are widely applied for load bearing for hard tissues their high fracture toughness and hardness. Consequently, there have been several previous works dealt with those composites. Sung et al. [69] synthesized HAp/yttrium-stabilized-zirconia (YSZ) nanocomposites by chemical co-precipitation method to enhance the mechanical properties of HAp. The results presented that YSZ improved the mechanical properties oh HAp, where flexural strength was ∼155 MPa and fracture toughness was ∼ 2.1MPm1/2. Es-saddik et al. [70] studied the effect of the sintering temperature on the mechanical properties, microstructure and densification of HAp/10% zirconia composite. The results demonstrated that the mechanical properties of the composite were very sensitive to the sintering temperature and densification process.
Numerous works fabricated mesoporous silica/HAp nanocomposites for different biomedical applications. Firouzjaei et al. [71] designed a biocompatible nanocarrier based on mesoporous silica/HAp to improve the bioavailability of piperine and studied its anticancer activity, and their results showed that piperine-loaded nanocarrier reduced MCF-7 breast cancer cells. Aghaei et al. [72] synthesized nanocomposite of mesoporous silica MCM-48/HAp as ibuprofen drug delivery system. Song et al. [73] used mesoporous silica/Hap composites for coating of gold nanorods as a multi-responsive drug delivery system. Such composites showed high loading efficiency and pH responsive of drug release. Yamada and Tagaya [74] investigated hydration and protein adsorption on mesoporous silica/HAp composites, where amounts of protein adsorption were high at the biological concentrations. Guo et al. [75] loaded miR-34a on implant wire coated with mesoporous organosilica/HAp for acceleration of bone fracture healing. The results displayed that this functionalized coated wire accelerated fracture healing.
Each type of biomaterials possesses advantages and disadvantages summarized in Table 2. Therefore, different properties can be tailored for specific application by selecting and combined suitable materials.
Methods used to fabricate tissue engineering scaffolds
There have been several techniques used and developed to fabricate tissue engineering scaffolds. The properties required for scaffold determine fabrication method that will be applied. The scaffold fabrication techniques can be subdivided into two major techniques: conventional techniques and rapid prototyping (RP) one. Figure 9 shows schematic presentation of techniques usually used for scaffold fabrication.
Conventional fabrication techniques
Freeze-Drying
Freeze-drying or lyophilization process contains a first material phase (such as polymer) dissolved in a second solvent phase, and this solution is cooled below the freezing point causing freezing of solvent to a solid state. The solid solvent is evaporated by sublimation process to form the first phase with numerous interconnected pores [76]. Figure 10 shows an example of fabrication of gelatin/chitosan/bioglass composite scaffolds by freeze-drying technique. The advantage of this method is using of water as a solvent instead of an organic solvent which resulting in decrease of scaffold contamination with toxic molecules which may causes cell death when the scaffold seeded with those cells.
Example of fabrication of gelatin/chitosan/bioglass composite scaffolds by freeze-drying technique. This figure is reproduced from [77] under an open access license distributed under the terms and conditions of the Creative Commons Attribution License, Copyright
Solvent casting and particle leaching
In this method, salt particles with specific size distributed uniformly in the polymer solution. After evaporation of solvent, polymer matrix including salt particles is formed. The salt grains are leached out by immerse polymer matrix in water forming a highly porous structure. The advantage of this technique is that the pore size of resulted scaffold can be tailored to be appropriate for cell growth, but it is suitable for 3D porous thin membranes only not for thick scaffold [78].
Gas Foaming
Inert gas such as CO2 or N2 is used to pressurize modeled polymer until full it saturated with gas bubbles. The porosity of resulted scaffold reach to 85%, and pore size is in the range 30–700 µm [79].
Electrospinning
Electrospinning is a technique used to fabricate nanofibrous scaffold from a solution by applying high voltage. The high voltage results in liquid charging and overcomes the liquid surface tension which causes elongation of liquid droplets to nanofibers. The standard electrospinning device contains spinner with a syringe pump, power supply of high-voltage, metallic needle and static or rotating grounded collector (Fig. 11). The solvent evaporates in this process, and jet is solidified to form into a nonwoven fibrous membrane [80].
Electrospinning device setup. This figure is reproduced from [81] under an open access license distributed under the terms and conditions of the Creative Commons Attribution License, Copyright
Thermal-induced phase separation
In this method, polymer solution is freeze causing its separation into two phases: polymer-rich phase and polymer-poor phase (usually solvent crystals). A porous scaffold can be obtained by extraction of solvent crystals by another solvent (such as ethanol) possesses lower freezing temperature at the same temperature that polymer solution freezes [80]. This technique can be used for preparation of thermoplastic crystalline polymer scaffold, and this low temperature is usually suitable to incorporate biomolecules within scaffold material.
Rapid prototyping (RP)
Rapid prototyping (RP) techniques or solid free-form fabrication (SFF) are computer-aided techniques. They directly fabricate the object made by computer-aided design (CAD) using computer without needing specific tools or devices. Plastic, wood, ceramic and metal object can be produced by these techniques by slicing it into layers using special computer software [82]. RP enables to fabricate scaffold with very precise structure, as well as, the scaffold shape and dimensions cane tailored according to the patient requirements. The following techniques are the basic techniques in RP techniques.
Stereolithography (SLA)
Stereolithography (SLA) technique basically prints layer-by-layer the object using ultraviolet (UV) curable polymer (photopolymer). SLA device composed of photopolymer container, linear actuator, UV laser source and dynamic mirror system (Fig. 12). The process carries out by solidification of a photosensitive liquid resin as successive layers, and then, the object is collected and uncured resin is removed, and finally, the scaffold is subjected to UV light for complete polymer curing.
Different types of rapid prototyping techniques. Reproduced with permission from [84], Copyright
Fused deposition modeling (FDM)
The thermoplastic biopolymers such as poly(ε-caprolactone) (PCL) are usually used in FDM technique. In this technique, a solid thermoplastic polymer filament is pushed through a hot extrusion nozzle and extruded due to its melting and deposited to form the required scaffold shape [83] (Fig. 12). The main drawback in this technique is using of polymer filament of definite diameter to go through nozzle, as well as, it is limited to specific biodegradable polymers.
Selective laser sintering (SLS)
In this technique, powder material is almost used. The laser beam controlled by the computer is selectively sintering of powder particles in certain points. The laser fuses the powder particles together by heating them to temperature below the material boiling point (sintering) or above its boiling point (melting). Figure 12 presents the process of SLS. This technique is suitable for fabrication of scaffolds of high mechanical strength such as ceramic and metal scaffolds which can be used for load bearing applications.
Three-dimensional printing (3DP)
3DP technique is similar to selective laser sintering (SLS), but the powder particles bind together with a solution (inkjet) as shown in Fig. 12. This method is desirable for ceramic, metal and ceramic/metal composite materials, and it is suitable for materials combined with biomolecules because the temperature is not applied in this method unlike SLS [81].
Bioprinting is one type of 3D printing, and it is a direct printing process to fabricate a biological pattern using cells, biomolecules and biodegradable materials [85] (Fig. 12). It can be classified into two kinds: acellular and cellular bioprinting. In an acellular bioprinting, the scaffold is fabricated without cells, while the cellular one, cells and other bioagents are included during printing process to obtain living tissue constructs. Currently, there are different types of 3D bioprinting: microextrusion, inkjet printing and laser-assisted and are the most commonly used methods for deposition and patterning of biological materials [85].
RP techniques, specifically 3D printing, can be combined with other techniques, such as electrospinning, to fabricate scaffolds with hierarchical structure. The filament diameter of scaffolds fabricated by extrusion-based 3D printing is usually above microns which is not similar to the submicron size of extracellular matrix fibers. Therefore, incorporation of submicron fibrous structure within the scaffold can mimic extracellular matrix surrounding the cells. As mentioned before, electrospinning technique produces nanofibers, and it can be provided such fibers on the scaffold surface or within it. Kim et al. [86] fabricated scaffolds based on poly(ε-caprolactone) (PCL) with alternating layers of micrometer strands and nanofibers by combining 3D printing and electrospinning. They studied the effect of this scaffold fabrication method against the chondrocytes, and they found that the scaffolds were significantly improved the cell growth. Similarly, Yu et al. [87] synthesized PCL/gelatin scaffolds by incorporating PCL/gelatin nanofibers fabricated by electrospinning in PCL scaffold prepared by 3D printing. The results showed that the composite scaffolds improved the proliferation and infiltration of MC3T3-E1 cells. Vyas et al. [88] fabricated PCL scaffolds by 3D printing including highly aligned electrospun fibers. Also, this hierarchical structure increased human adipose-derived stem cells proliferation and aligned the cells with an elongated morphology.
Recent applications of biomaterials in tissue engineering
Bone tissue regeneration
Bone tissue is mainly composed of collagen protein molecules and hydroxyapatite (HAp) mineral. Collagen molecules twine together to form tropocollagen strands align to form repeated intersecting districts separated by small spaces filled with HAp nanocrystals forming mineralized strands (Fig. 13). The mineralized strands join together to build a fibril. The fibers and cylindrical lamellae form high-level osteon constructions in the compact bone. A Haversian canal, which has a blood providing function to the bone tissue, goes through the center of every osteon. The macroscopic bone structure is mainly composed of two types of tissue: the outer shell compact bone and interior trabecular or spongy bone [89].
Bone tissue structure. Reproduced from [90] under Creative Commons Attribution License.
Bone defects resulted from fracture, trauma, bone deformation, neoplastic disease and osteoporosis require reconstructing and regenerating this bone tissue to restore the structure and integrity of the bone function [91]. Accordingly, it is essential to replace the defected bone part with suitable graft distinguished by high biocompatibility, osteoinductivity, osteoconductivity, osseointegrativity and high mechanical properties [92]. Therefore, current trends in bone tissue engineering have concentrated on enhancement of biomaterials that mimic microstructure, biological and mechanical properties of bone tissue. Wu et al. [93] studied the effect of engineered porous architecture of Mg-substituted wollastonite scaffolds on the in vivo bone regeneration. They tailored scaffold pore size using the advances of stereolithography technique. They found that the scaffolds of 450 μm and 600 μm were assisted formation and remodeling of new bone tissues better than other pore sizes. Dellavia et al. [94] used Ti scaffold fabricated by selective laser sintering (SLS) for guided alveolar bone regeneration (Fig. 14) of severe posterior mandibular atrophy of 20 patients at the Clinical Unit of Oral Surgery, ASST Santi Paolo e Carlo (Milan, Italy). The scaffold meshes were filled with autologous bone (bone taken from the patient) and 50% of deproteinized bovine bone, and then, they covered with porcine collagen membrane, and finally, the scaffolds were implanted in the patients. The histological analyses showed that these Ti meshes could be regenerated the alveolar bone with no significant shape change in the alveolar bone. Omar et al. [95] fabricated 3D printed Ca-phosphate bioceramic implants including titanium frames implanted to reconstruct a large cranial defect in the ovine skull (Fig. 15). The results showed that the ceramic implant was stimulated bone regeneration, as well as, the ceramic implant surface converted to carbonated hydroxyapatite. They recommended that the human cranial defect reconstruction can be carried out by in situ regeneration using this Ca-phosphate implant.
a Ti-mesh produced by selective laser sintering (SLS) technique b Initial projection of the Ti-mesh on 3D model obtained by elaboration of patients. Adapted from [94], Copyright
Cranial implant used in the human skull defect. a The experimental bioceramic implant (calcium phosphate) interconnected by an additively manufactured titanium frame. b The titanium implant (control). c The BioCer cranial implant used in the human skull. This figure is reproduced from [95] under an open access license distributed under the terms and conditions of the his open access article is distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND), Copyright
Recently, biomimetic scaffolds are more effective for bone tissue engineering, because they outstand bone graft limitations, such as donor site morbidity and limited availability [96], where the improvement of osteogenesis and vascularization in bone regeneration process using a scaffold can be achieved by incorporating angiogenic and osteogenic factor (e.g., bone morphogenic proteins and vascular endothelial growth factor) in this scaffold [97,98,99]. Kim et al. [100] prepared collagen/β-tricalcium phosphate scaffolds loaded with human umbilical vein endothelial cells (HUVECs) and human adipose stem cells (hASCs) for bone regeneration. Their results acquired that these biomimetic scaffolds promoted the activity of angiogenesis and osteogenesis. Yang et al. [101] fabricated core–shell fibers from poly (3-hydroxybutyrate-co-4-hydroxybutyrate)/poly (vinyl alcohol) (P34HB/PVA) with human bone mesenchymal stem cells (hBMSCs) by electrospinning technique. Zhou et al. [102] combined autologous concentrated growth factor with silk fibroin/chitosan/nano-HAp to promote the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells for bone defect treatment. The cell viability results the combination of this growth factor with the scaffold group presented better adhesion, proliferation and osteogenic differentiation. Furthermore, in vivo test of this biomimetic scaffold in a rabbit radius critical bone defect model showed better proficiency in bone defect repair than other growth factor-free scaffolds.
Cartilage tissue regeneration
Cartilage is a flexible connective tissue present in many parts of the body, such as the intervertebral discs, ribs, joints, ear, nose and trachea. It absorbs shock during movements and keeps the body structure. The cartilage cell growth and tissue regeneration are limited due to cartilage is avascular tissue, which leads to hypoxic environments [103]. This results in capacity limitation of cartilage to restore itself. Cartilage is made up of specialized cells called chondrocytes. The cartilage is mainly composed of chondrocytes, which produce great quantities of extracellular matrix composed of proteoglycan, collagen fibers and elastin fibers (Fig. 16).

Adapted from from [107], Copyright© 2017, John Wiley and Sons
Schematic diagram of knee joint (left), articular cartilage and adjacent structures (right).
Cartilage harm and clinical degeneration are recorded for millions of patients worldwide, and it is limited to be regenerated due to absence of vascular tissue. And consequently, repair and regeneration of damaged cartilage tissue is a critical issue, so that it has drawn the interest in the biomedical field. Wang et al. [104] synthesized hydrogel based on gelatin methacrylamide (GelMA), ε-poly-l-lysine (EPL) and/or 3-Aminophenylboronic acid (PBA) and seeded with chondrocytes. They implanted synthesized hybrid hydrogels subcutaneously into the back of rat. In vitro results showed that the hydrogels induced excretion of further extracellular matrix and enhanced chondrogenic differentiation. In vivo findings demonstrated that the hydrogel supported the tissue regeneration of cartilage defects as shown in Fig. 17. Yaqiang et al. [105] synthesized scaffolds with hierarchical porous microstructure and based on bacterial cellulose/decellularized cartilage extracellular matrix for cartilage repair and regeneration. The prepared scaffolds exhibited excellent mechanical and shape-memory properties and water absorption. As well as, cell adhesion and proliferation were enhanced by the scaffolds. In vivo results showed that the scaffolds were promoted formation of neocartilage and tissue regeneration within the defect sites. Haghighi et al. [106] prepared gelatin/chitosan/silk fibroin scaffolds. Pore size of the scaffolds ranged from 100 μm to 300 μm, and the water wettability and degradation rate were attained appropriate for cartilage tissue regeneration. Moreover, the human chondrocytes were showed good cell adhesion and proliferation. Finally, the optimum scaffold was seeded with chondrocytes and in vivo tested in the rabbit, and the results presented formation of new cartilage tissue in the defect compared to the control group which did not implanted with the scaffold.
Photos of healing of cartilage defects in the rat treated with different hydrogels at 4-week post-operation. This figure is reproduced from [104] under an open access license distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License, Copyright
As mentioned before, the articular cartilage lacks self-regenerative cells ability, so providing of cartilage defects with chondrogenic cells is urgent for the cartilage defect repair. Therefore, there have been several types of cells combined and chondroinductive growth factors with scaffolds as a promising technique for cartilage tissue regeneration. Chondrocytes and mesenchymal stem cells (MSCs) have widely combined with scaffolds for chondral defect repair. Li et al. [108] prepared nanofibrous scaffold based on poly(ε-caprolactone) cultured with MSCs in the presence of TGF-β1 for cartilage tissue engineering. The results allocated that cells differentiated to a chondrocytic phenotype, and the chondrogenesis level for cell-seeded scaffold was higher than cells cultured without the scaffold. As well, the scaffold seeded with these cells possessed appropriate mechanical properties for cartilage tissue regeneration. In a similar work, da Silva et al. [109] seeded MSCs onto poly(ε-caprolactone) nanofiber scaffold, but they cultured them in a multichamber flow perfusion bioreactor to study their capability to give cartilaginous extracellular matrix. The results showed that such bioreactor increased the chondrogenic differentiation. Heirani-Tabasi et al. [110] synthesized injectable chitosan-hyaluronic acid hydrogel loaded with adipose-derived MSCs and treated with chondrocyte extracellular vesicles. They found that this treatment increased chondrogenic genes' expressions of SOX9 and COL2A1 and improved of Col II protein expression. Moreover, the results of in vivo test in a rabbit osteochondral defect model demonstrated that the cartilage regeneration ability of cell-combined hydrogel was greater than cell-free hydrogel. Rathan et al. [111] fabricated the bioprinting of cartilaginous tissues from cartilage extracellular matrix—functionalized alginate bioink support of MSCs. This bioink enhanced robust chondrogenesis.
Skin tissue regeneration
Skin is the largest and important organ in the body. It protects the inner organs from the infections and harmful cosmetic rays, controls body temperature and performs a serious role vitamin D synthesis. Skin is composed of three layers: (1) an outer cell-rich epidermis, (2) an intermediate extracellular matrix-rich dermis which mainly composed of fibroblasts and (3) an inner layer of fat-rich adipocytes (Fig. 18).
Dangerous damage to the skin may consequently be life-minatory. Wound healing and skin regeneration need integrated biological and molecular fields including inflammation, proliferation and remodeling. Currently, there are a wide array of skin ointments, wound dressings and medical devices, but, skin regeneration and wound healing continue a clinical dispute, specifically in the diabetic patients, old people and burned patients. Acceleration of wound healing requires the surface wound be kept moist, instead of being subjected to air [112]. Traditional treatment and healing of small wounds used wound dressings include gauze and tulle. But, they can cause a pain upon removal due to their adherence to the wound bed. Recently, the modern wound dressings can enhance the wound healing [113]. For extensive deeper, larger and chronic wounds, skin tissue regeneration is required in this case. Therefore, there is still need to find more efficacious methods for wound healing without leaving of disfiguring scars termed. The scaffold is preferred for cell proliferation and tissue regeneration. Beside non-toxicity, biodegradability and porous structure, the scaffold should have antibacterial properties to prevent the infection. It is important to mention that wound healing using biomaterials tissue regeneration approach still a major interest of research worldwide.
Skin scaffolds are usually prepared from either natural polymers or synthetic polymers. Chen et al. [114] fabricated PLA nanofibers loaded with adipose-derived stem cells by protein freeze-drying and emulsion electrospinning technologies. They in vitro studied the migration fibroblasts by cell direct contact culture with this nanofiber. The results showed that the nanofibers accelerated fibroblasts migration rate. The in vivo wound healing experiments were performed by implanting the nanofiber in a full thickness skin defect of mice for 15 days. The nanofiber significantly enhanced wound healing and promoted skin tissue regeneration. Composites have also been used for skin regeneration. Sun et al. [115] prepared coaxial PLA, PLGA/fibrinogen and PLGA/collagen nanofibrous scaffolds by electrospinning method for wound healing and skin regeneration. Fibrinogen and collagen I used in this study as two essential extracellular matrix proteins, they incorporated into the shell and the core of nanofibers, respectively, to mimic the consecutive look of fibrinogen and collagen I in the wound healing procedure. They used adipose-derived mesenchymal stromal cells to compare their regulation with that of scaffolds nanofibers. As well as, in vivo wound healing was carried out in by implanting different scaffolds in full thickness skin defects of rat. The results showed that the scaffolds obviously enhanced the immunomodulatory secretion of adipose-derived mesenchymal stromal cells. As well as, the scaffolds efficiently improved wound repair in the skin defects of rats. Chitosan/gelatin can be fabricated successfully for skin regeneration, and the best suitable ratio between chitosan and gelatin for soft and skin tissue regeneration 3:10 from the side of mechanical properties [116]. Chitosan/collagen composites also applied for wound dressing and skin regeneration. Collagen is preferred for skin substitution because it acts as a 70% of dry skin mass, and chitosan was added to improve its mechanical properties. Sarkar et al. [117] fabricated hierarchical nano/microfibrous chitosan/collagen scaffold for skin tissue engineering. They made chitosan fibrous scaffold by electrospinning followed by immersing it in collagen solution and cross-linking of both polymers. The produced structure is similar to the extracellular matrix structure. Moreover, metal nanoparticles such as silver, copper, zinc and gold can be added for wound dressings due to their low toxicity and antibacterial properties [118].
Growth factors, such as TGF-β, vascular endothelial growth factor and epidermal growth factor, and cells, such as stem cells, mesenchymal stem cells and adipose stem cells (MSCs) and epithelial cells, have been proposed to be added to the skin substitutes to enhance skin regeneration. Maged et al. [119] prepared chitosan hydrochloride/collagen/β-glycerol phosphate, /carboxymethyl cellulose scaffold incorporated rosuvastatin calcium and loaded it with MSCs. They studied its healing ability of wounds induced in rat models. Their results showed the wound size was closed completely after 7 days. Sahoo et al. [120] loaded fibroblast growth factor in poly(lactic-co-glycolic) acid fibers by uniaxial and coaxial electrospinning method. They found that both fibers supported attachment and proliferation from bone marrow stem cells in high levels. Zandi et al. [121] developed bilayer scaffolds based on gelatin nanofibers (act as the dermis layer) and Laponite-gelatin-methacrylate composite hydrogel (acted as the epidermis matrix) and loaded them with epidermal growth factors. It was shown that the scaffolds loaded with growth factors stimulated skin regeneration completely after 14 days in Wistar albino rats model.
Recently, wound healing can be stimulated by electricity, in which external electrical field is applied to stimuli the wound tissue to grow [122]. Therefore, conductive materials, such as conductive polymers (e.g., polythiophene, polypyrrole and polyaniline), metals, carbon nanomaterials (single-walled and multi-walled carbon nanotubes, graphene oxide) and metal oxides, have been incorporated into substitutes used for skin regeneration. Beside their ability to facilitate electrical stimulation of wounds, the release of loaded biological agents can be controlled by an external electrical field. Fu et al. [123] synthesized electrical conductive composites based on cellulose and polypyrrole or poly(3,4-ethylenedioxythiophene) and doped with copper and zinc ions by electropolymerization onto platinum-coated cellulose substrates in the existence of sodium dodecyl sulfate. The results demonstrated that both composites showed good mechanical properties, and their viability against immortalized human keratinocytes supported showed good cell attachment and proliferation. Aycan et al. [124] fabricated electroconductive composites based on sodium alginate/gelatin/hyaluronic acid/reduced graphene oxide and loaded them with ibuprofen anti-inflammatory agent for wound dressing. Their results presented that the composites of high conductivity value reinforced better cell attachment and proliferation and anti-inflammatory effect.
Liver tissue regeneration
Liver possesses high complex microarchitecture, and it is one of complex organs in the body. Liver is a multicellular architecture; it is mainly composed of hepatocytes (80–85% of total liver cells), extracellular matrix, liver sinusoidal endothelial cells and hepatic stellate cells (Fig. 19). Numerous approaches have been performed using a wide range of biomaterials for liver tissue engineering, but it was very difficult to achieve this task, that is because of the precise configuration of the liver cells. In the current review, recent progress of using biomaterials for liver tissue engineering is presented here. Fabrication of integrated liver cells and biomaterials structure similar to the liver architecture is a serious challenge, and it almost need advanced techniques which have capability to fabricate scaffolds with precise micro- or nanostructures. Nguyen et al. [125] fabricated scaffolds containing hepatocytes and non-parenchymal cells were functionally active. As well as, they reported them as medical patterns of drug-induced liver harm. Ghahremanzadeh et al. [126] modified the surface of polycaprolactone/chitosan scaffold nanofiber by galactosylation of chitosan to be used in liver tissue engineering using electrospinning technique. This modification was increased hydrophilicity of the scaffolds. Culturing the scaffolds with the human hepatic (HepG2) cells showed that the presence surface modification of nanofibers by galactose was significantly enhanced cell growth and proliferation.
Ultrastructure of liver tissue. Adapted from [127], Copyright
Moniruzzaman et al. [128] developed new biocompatible hydrogel scaffolds based on novel gelatin functionalized with glycidyl methacrylate, beside this, the scaffolds were encapsulated Huh-7.5 (human hepatocarcinoma) cells for cell compatibility and hepatocyte specialized functions. The gelatin derivative hydrogel scaffolds were characterized by large mesh sizes which provided sufficient hole for the cell proliferation and functionalities of Huh-7.5 cells which results in faster unification of the cell aggregates over the time. Muxin et al. [129] fabricated macro human liver polycaprolactone (PCL) scaffolds with interconnected flow channels by the selective laser sintering (SLS) technique. Polyglycolic acid (PGA) microparticles were used as porogens to ensure the printing of flow channels. The scaffolds were seeded with Hep G2 cells, and then it endothelized with endothelial cells grown under aspiration of culture medium for 10 days. Finally, the scaffold covered with platelet-rich plasma for evaluation of hemocompatibility (Fig. 20). The scaffolds were showed a distinctly glucose metabolism, different albumin production and lactate production. These results concluded that the scaffold with interconnected flow channels can be aspirated with whole blood and avoiding the restriction of blood compatibility for liver tissue engineering. Similarly, Xia et al. [130] developed bioartificial liver developed using cylindrical bioreactor contained 12 double-sandwich culture plates containing hepatocytes, each culture plate coated at the bottom by collagen-coated polyethylene terephthalate and the top was coated with a porous and nonporous collagen-coated polyethylene terephthalate membrane. Such bioreactor was able to house 100 million. The results showed that hepatocytes cultivated in this bioreactor were capable to keep liver-specific phenotype and functions for 7 days.
a Schematic presentation of PGA polymer leaching to fabricate PCL macro scaffold fabricated by SLS technique, b schematic illustration of aspiration culture setup, c configuration of aspiration culture system. Reproduced from [129] under an open access license distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license Copyright
In the recent decades, delivery of cells (e.g., hepatocytes, hepatic and non-hepatic stem cells) by suitable biomaterials provides a promising solution to replace damaged hepatocytes and stimulate healthy ones and restore the liver function. Shang et al. [131] co-cultured endothelial cells and hepatocytes with scaffold based on hyaluronic acid and galactosylated chitosan using a freeze-drying technique. Their scaffold showed good viability with both cell types, and it recommended to be used as a matrix for the co-culture of endothelial cells and hepatocytes in liver tissue engineering. Lau et al. [132] encapsulated murine induced pluripotent stem cells (miPSCs) and embryonic stem cells (ESCs) by 3D microporous alginate hydrogel for hepatic lineage differentiation. The results demonstrated that cells differentiated in hydrogel better than cells monolayer, and they showed higher urea and albumin productions which are marks of liver function efficiency. Brown el al. [133] studied influence of incorporation of type I collagen or fibronectin onto electrospun PLGA on accommodation and function of hepatocytes. The results showed that the cells maintained their long-term in vitro existence and stimulated function in PLGA electrospun coupled with type I collagen.
Vascular tissue regeneration
Vascular diseases, especially cardiovascular diseases, are considered one of mean death causes worldwide. To date, replacement of small diameter blood vessels is performed autologous harvest. Nevertheless, vessels autografts show some drawbacks, such as scarce availability and morbidity [134]. And consequently, using of tissue engineering concept to fabricate vascular-like materials similar to vascular structure and its biological and mechanical properties have been the best choice for replacement of blood vessels [135]. The basic approach for vascular tissue engineering involves synthesis of scaffolds of suitable mechanical properties, good adhesion, proliferation and differentiation of vascular cells, where at the beginning, porous scaffold is fabricated in the tubular shape, then specific cells are growing onto the scaffold. The scaffold quickly degrades, and the cells secrete extracellular matrix proteins, the construct is incubated in a special bioreactor for maturation, and kept in a buffer solution up to the in vivo implantation.
Geng et al. [114] prepared hydrogel tubular scaffolds composed of heparin-modified poly(ε-caprolactone) by electrospinning technique. They in vivo tested the scaffolds by implanting them in the rat abdominal aorta. The results showed that the hydrogel scaffolds showed good vascular regeneration, and the scaffold aneurysm incidence rate was decreased. And consequently, the synthesized hydrogel scaffolds were obviously showed the in vivo vascular regeneration property. Jiang et al. [136] incorporating carbon nanotubes/polycaprolactone/gelatin scaffold yarns for vascular regeneration by electrospinning of polycaprolactone/gelatin polymer into a bath of carbon nanotubes dispersion, and then interweaving the yarns into a textile and linking it with gelatin. The obtained scaffolds presented mechanical properties similar to native vessels, as well as, they improved cell proliferation. Dimopoulos et al. [137] prepared small-caliber multilayer polycaprolactone vascular scaffolds for cardiovascular tissue engineering using electrospinning technique (Fig. 21). A multilayer design was selected to mimic the artery wall structure. The axial elastic modulus of the obtained scaffold was 18 ± 3 MPa axially, which is similar to that of natural arterial walls, as well as, the radial compliance (5.04 ± 0.82%) value was located within the physiological pressure array. Finally, cytotoxicity evaluation of polymeric scaffolds with the human cerebral microvascular endothelial cells bared that the scaffolds were exhibited > 80% of cell viability.
Multilayered polycaprolactone vascular scaffold. Reproduced from [137] under an open access license distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License Copyright
Recent trends and challenges of biomaterials for tissue regeneration
This article reviewed preparation and application of different biomaterials for tissue regeneration applications and it highlighted the previous works concerned with using of biomaterials to regenerate different types of tissues, such as bone, skin, liver and vessels. As mentioned throughout this review, the historical development of synthetic biomaterials has been gone through three generations with respect to reaction of such materials toward surrounding tissues. First generation biomaterials are biologically inert materials, second generation is bioresorbable and bioactive materials and third generation is cellular stimulating materials. Recently, biomaterials are developed to stimulate cell and regenerate tissue, and there have been various attempts made to prepare biomaterials with the novel properties for a variety of tissue engineering and tissue regeneration. Accordingly, such materials have been developed in two issues: first one is the development of composition, morphology and surface roughness, as well as, modification and functionalization of biomaterials; second one is development of methods and technologies of preparation of such materials.
As mentioned before, a scaffold has an important role in the process of tissue engineering procedures, and its complex shape is a challenge, which limits the progress of developing of new novel templates. In order to overcome this challenge, technology of preparation of such materials should be developed through the collaboration between engineers, material scientists, biologist and clinicians. Therefore, most of the recent researches are focusing on optimizing the materials and methods of preparation of substrate with complex shape. Recently, rapid prototyping techniques are the most promising methods to prepare scaffolding materials with precise shape. However, development of scaffolds seeded with specific cells remains a main critical issue, because cells will not survive without an adequate blood supply. And so, the recent fabrication techniques are not able to produce scaffolds mimic the hierarchical structure of some complex tissues, such as liver, lungs and small diameter vessels due to a shortage to tailor scaffold geometry, difficulty to encapsulated cells and lacking to control cell patterning [138,139,140]. Nevertheless, 3D bioprinting technology can be considered the most appropriate technique among other techniques. As mentioned before, 3D bioprinting is a process to fabricate cell-loaded bioinks into tissues and organs from 3D digital models [141]. It allows to print cells or biologically derived materials to fabricate scaffolds and tissues with proper biomechanical, biochemical and physiological properties in connection with patient-specific needs [142]. Therefore, 3D bioprinting is a promising nominee to be applied for fabrication of complex structures. Figure 22 shows the applications of 3D bioprinting in different organs in the human body.
Using of 3D bioprinting in different organ systems of the human body. Adapted from [143] under an open access license distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license Copyright
There has been a rapid increase in the number of patients waiting for organ transplantation, where there were about 122,000 patients staying for organ transplantation in USA in 2016 [144]. Consequently, 3D bioprinting has a great promise to provide fully functional organs; specifically substantial progress has been achieved in this way. This was evidenced from multiplication of the publication number of 3D bioprinting to 3300% from 2000 to 2015 [145]. We presented in this review application of biomaterials in regeneration of some specific tissues. Herein, the recent achievements and challenge in the tissue regeneration and replacement of some human body parts with synthetic biomaterials using 3D bioprinting are still restricted.
Despite there have numerous successful fabricated scaffolds or semi-biological skeletal human parts been reported by bioprinting, there are still challenges to obtain fully functional skeletal organ. The foremost challenge of bone and cartilage regenerations using 3D bioprinting is keeping cell viability by ensuring flow of nutrients and growth factors inside the 3D printed structure [146]. Bioprinting of structure with microarrays [147] and bioprinting of adapted precursor used as an organogenesis template [148] are considered recent trends to overcome this drawback. Moreover, there is a zonal variation in the bone and cartilage structure and cell density which is a critical issue in bioprinting of the biomimetic structure is not achieved yet. Nevertheless, using of hydrogels with tailored cell density and mechanical strength can present a promising solution in this side. Finally, some types of cells, such as chondrocytes, are limited in availability [149]. This cell unavailability challenge can be overcome by using of stem cells.
The main obstacle of bioprinting of the artificial liver is the availability shortage of hepatic cells [150], and gradual losing of their function after ex vivo culturing [151]. Alternatively, stem cells can solve this limitation. Another important organ that aimed to be fabricated by bioprinting is the kidney. However, the kidney architectural complexity and unavailability of primary are the main challenges of printing of full functional kidneys [152]. Where, formation of nephrons alike to the native kidney is very difficult. Furthermore, a complex renal architecture is considered a great challenge to gain a fully functional kidney by bioprinting [153]. The suggested solution of this challenge is using of tissue spheroids bioink which can be self-assembled to form the kidney tissue. It is expected to be a potential approach in the future.
Bioprinting of some cardiovascular system parts (heart and blood vessels) is currently possible in the market, such as bioprinting blood vessels and cardiac patches for treatment of myocardial infarction. The recent efforts concentrated replacement of the bioprosthetic and mechanical valves bioprinted valves based on patients own cells. Preparation of an ideal cardiac bioink with suitable rigidity and cell microenvironment remains a challenge [154]. An attracting future direction is elaboration of in situ controllable crosslinked bioinks to control different physicochemical properties of the final bioprinted cardiac tissues. Nonetheless, bioprinting of the fully functional heart remains very distant from reality.
Tissue engineering scientists' efforts are still seeking to achieve a dream that is far from being achieved at the present time, which is the possibility of manufacturing human organs in the laboratory. But they have hopefulness to achieve this in the future. Who knows, the day may come when we see specialized departments in hospitals, or perhaps private companies, working on the manufacture of human organs, like liver, kidney and heart, to be replaced in the patient with the required specifications and characteristics.
Conclusion
This review presented types and progress of biomaterials in the tissue engineering field. Tissue engineering depends on the scaffolding biomaterials, and their composition and complex shape are a challenge. Biomaterials used in tissue engineering are classified as a third generation of biomaterials which can induce cell growth. The review showed different types of biomaterials, such as bioceramics, bioactive glasses, synthetic and natural polymers, and their derived composites. Each class of biomaterials is preferred for a specific part in the body, for example, bioceramics are suitable for the human hard tissue. Moreover, complex shape, precise architecture and desired mechanical strength of the scaffold are considered a challenge to obtain ideal scaffold. In order to overcome this challenge, technology of preparation of such materials should be developed through the collaboration between engineers, material scientists, biologist and clinicians. Recently, 3D bioprinting rapid prototyping techniques are the most promising methods to prepare scaffolding materials with precise shape. However, bioprinting technique still has a limitation to obtain fully functional human organs, and there are efforts to fabricate a complete organ, but, they have hopefulness to achieve this in the future.
Authors information
The author is Associate Professor in Biomaterials Department, National Research Centre.
Availability of data and materials
Not applicable.
References
Langer R, Vacanti JP (1993) Tissue engineering. Science I 993:260
Hench LL, Polak JM (2002) Third-generation biomedical materials. Science 295:1014–1017
Williams D (2004) Benefit and risk in tissue engineering. Mater Today 7:24–29
Hutmacher DW (2001) Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci Polym Ed 12:107–124
Asadian M, Chan KV, Norouzi M, Grande S, Cools P, Morent R et al (2020) Fabrication and plasma modification of nanofibrous tissue engineering Scaffolds. Nanomaterials 10:119
Hench LL, Splinter RJ, Allen W, Greenlee T (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5:117–141
Hench LL (1998) Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res 41:511–518
Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF et al (2011) Bioactive glass in tissue engineering. Acta Biomater 7:2355–2373
Hench L, Paschall H (1974) Histochemical responses at a biomaterial’s interface. J Biomed Mater Res 8:49–64
Hench LL, Andersson O (1993) Bioactive glasses. Adv Ser Ceram 1:41–62
Kokubo T, Ito S, Huang ZT, Hayashi T, Sakka S, Kitsugi T et al (1990) Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J Biomed Mater Res 24:331–343
Andersson Ö, Liu G, Kangasniemi K, Juhanoja J (1992) Evaluation of the acceptance of glass in bone. J Mater Sci Mater Med 3:145–150
Yao A, Wang D, Huang W, Fu Q, Rahaman MN, Day DE (2007) In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J Am Ceram Soc 90:303–306
Fu Q, Rahaman MN, Fu H, Liu X (2010) Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J Biomed Mater Res Part A 95:164–171
Marion NW, Liang W, Liang W, Reilly GC, Day DE, Rahaman MN et al (2005) Borate glass supports the in vitro osteogenic differentiation of human mesenchymal stem cells. Mech Adv Mater Struct 12:239–246
El-Meliegy E, Farag MM, Knowles JC (2016) Dissolution and drug release profiles of phosphate glasses doped with high valency oxides. J Mater Sci Mater Med 27:108
Hench LL (2006) The story of Bioglass®. J Mater Sci Mater Med 17:967–978
Vallet-Regí M, Ragel C, Salinas AJ (2003) Glasses with medical applications. Eur J Inorg Chem 2003:1029–1042
Li R, Clark A, Hench L (1991) An investigation of bioactive glass powders by sol-gel processing. J Appl Biomater 2:231–239
De Aza P, Guitian F, Merlos A, Lora-Tamayo E, De Aza S (1996) Bioceramics—simulated body fluid interfaces: pH and its influence of hydroxyapatite formation. J Mater Sci Mater Med 7:399–402
Brinker CJ, Scherer GW (2013) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, Cambridge
McDonagh C, Sheridan F, Butler T, MacCraith B (1996) Characterisation of sol–gel-derived silica films. J Non-Cryst Solids 194:72–77
Stookey S (1959) Catalyzed crystallization of glass in theory and practice. Ind Eng Chem 51:805–808
Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K (2001) Bone induction by porous glass ceramic made from Bioglass®(45S5). J Biomed Mater Res 58:270–276
Baino F, Fiume E, Barberi J, Kargozar S, Marchi J, Massera J et al (2019) Processing methods for making porous bioactive glass-based scaffolds—a state-of-the-art review. Int J Appl Ceram Technol 16:1762–1796
Fu Q, Rahaman MN, Bal BS, Brown RF (2010) Preparation and in vitro evaluation of bioactive glass (13–93) scaffolds with oriented microstructures for repair and regeneration of load-bearing bones. J Biomed Mater Res Part A 93:1380–1390
Fu Q, Rahaman MN, Bal BS, Brown RF, Day DE (2008) Mechanical and in vitro performance of 13–93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater 4:1854–1864
Keaveny T, Hayes W, Hall B (1993) Bone. A treatise, vol 7: Bone growth; B
Fung Y-C (2013) Biomechanics: mechanical properties of living tissues. Springer, Berlin
Baino F, Verné E, Vitale-Brovarone C (2009) 3-D high-strength glass–ceramic scaffolds containing fluoroapatite for load-bearing bone portions replacement. Mater Sci Eng C 29:2055–2062
Farag MM, Rüssel C (2012) Glass-ceramic scaffolds derived from Bioglass® and glass with low crystallization affinity for bone regeneration. Mater Lett 73:161–165
Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J et al (2013) Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 34:422–433
Gogele C, Wiltzsch S, Lenhart A, Civilleri A, Weiger TM, Schafer-Eckart K et al (2021) Highly porous novel chondro-instructive bioactive glass scaffolds tailored for cartilage tissue engineering. Mater Sci Eng, C Mater Biol Appl 130:112421
Driessens F (2018) Formation and stability of calcium phosphates in relation to the phase composition of the mineral in calcified tissues. In: Bioceramics of calcium phosphate. CRC Press, Boca Raton, pp 1–32
Bohner M (2010) Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater 20:1–12
Wei J, Jia J, Wu F, Wei S, Zhou H, Zhang H et al (2010) Hierarchically microporous/macroporous scaffold of magnesium–calcium phosphate for bone tissue regeneration. Biomaterials 31:1260–1269
Zhang J, Zhou H, Yang K, Yuan Y, Liu C (2013) RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials 34:9381–9392
Wagh AS, Primus C (2006) Method and product for phosphosilicate slurry for use in dentistry and related bone cements. Google Patents
Farag MM, Yun H-S (2014) Effect of gelatin addition on fabrication of magnesium phosphate-based scaffolds prepared by additive manufacturing system. Mater Lett 132:111–211
Jongman Lee MMF, Park EK, Lim J, Yun H-s (2014) A simultaneous process of 3D magnesiumphosphate scaffold fabrication and bioactive substance loading for hard tissue regeneration. Mater Sci Eng C 36:52–260
Moseke C, Saratsis V, Gbureck U (2011) Injectability and mechanical properties of magnesium phosphate cements. J Mater Sci Mater Med 22:2591–2598
Tamimi F, Le Nihouannen D, Bassett DC, Ibasco S, Gbureck U, Knowles J et al (2011) Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomater 7:2678–2685
Yu Y, Wang J, Liu C, Zhang B, Chen H, Guo H et al (2010) Evaluation of inherent toxicology and biocompatibility of magnesium phosphate bone cement. Colloids Surf, B 76:496–504
Mestres G, Ginebra MP (2011) Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater 7:1853–1861
Maguire ME, Cowan JA (2002) Magnesium chemistry and biochemistry. Biometals 15:203–210
Wacker WE (1980) Magnesium and man. Harvard University Press, Cambridge
Elin RJ (2010) Assessment of magnesium status for diagnosis and therapy. Magnes Res 23:S194–S198
Welch AA, Skinner J, Hickson M (2017) Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: cross-sectional findings from the UK Biobank cohort. Nutrients 9:1189
Lee J, Farag MM, Park EK, Lim J, Yun HS (2014) A simultaneous process of 3D magnesium phosphate scaffold fabrication and bioactive substance loading for hard tissue regeneration. Mater Sci Eng, C Mater Biol Appl 36:252–260
Götz L-M, Holeczek K, Groll J, Jüngst T, Gbureck U (2021) Extrusion-based 3D printing of calcium magnesium phosphate cement pastes for degradable bone implants. Materials 14:5197
Farag M, Liu H, Makhlouf A (2021) New nano-bioactive glass/magnesium phosphate composites by sol–gel route for bone defect treatment. SILICON 13:857–865
Abdelraof M, Farag MM, Al-Rashidy ZM, Ahmed HY, El-Saied H, Hasanin MS (2022) Green synthesis of bioactive hydroxyapatite/cellulose composites from food industrial wastes. J Inorg Organomet Polym 32(12):4614–4626
Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S (1998) Fibrillar structure and mechanical properties of collagen. J Struct Biol 122:119–122
Lou X, Chirila TV (1999) Swelling behavior and mechanical properties of chemically cross-linked gelatin gels for biomedical use. J Biomater Appl 14:184–191
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Kim HW, Song JH, Kim HE (2006) Bioactive glass nanofiber–collagen nanocomposite as a novel bone regeneration matrix. J Biomed Mater Res, Part A 79:698–705
Bae WJ, Min KS, Kim JJ, Kim JJ, Kim HW, Kim EC (2012) Odontogenic responses of human dental pulp cells to collagen/nanobioactive glass nanocomposites. Dental Mater 28:1271–1279
Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR et al (2011) Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 32:8915–8926
Mozafari M, Moztarzadeh F, Rabiee M, Azami M, Maleknia S, Tahriri M et al (2010) Development of macroporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tissue engineering. Ceram Int 36:2431–2439
Mozafari M, Rabiee M, Azami M, Maleknia S (2010) Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds. Appl Surf Sci 257:1740–1749
Lei B, Wang L, Chen X, Chae S-K (2013) Biomimetic and molecular level-based silicate bioactive glass–gelatin hybrid implants for loading-bearing bone fixation and repair. J Mater Chem B 1:5153–5162
Zhou Z, He S, Ou B, Huang T, Zeng W, Liu L et al (2014) Influence of nano-bioactive glass (NBG) content on properties of gelatin-hyaluronic acid/nbg composite scaffolds. J Macromol Sci Part B 53:1145–1155
Gönen SÖ, Taygun ME, Aktürk A, Küçükbayrak S (2016) Fabrication of nanocomposite mat through incorporating bioactive glass particles into gelatin/poly (ε-caprolactone) nanofibers by using Box-Behnken design. Mater Sci Eng, C 67:684–693
Sharifi E, Azami M, Kajbafzadeh AM, Moztarzadeh F, Faridi-Majidi R, Shamousi A et al (2016) Preparation of a biomimetic composite scaffold from gelatin/collagen and bioactive glass fibers for bone tissue engineering. Mater Sci Eng, C Mater Biol Appl 59:533–541
Yu X, Shen G, Shang Q, Zhang Z, Zhao W, Zhang P et al (2021) A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat. Int J Biol Macromol
Wang S, Umrath F, Cen W, Reinert S, Alexander D (2021) Angiogenic potential of VEGF mimetic peptides for the biofunctionalization of collagen/hydroxyapatite composites. Biomolecules 11:1538
Bhushan M, Mohapatra D, Kumar Y, Kasi VA (2021) Fabrication of novel bioceramic α-Fe2O3/MnO nanocomposites: study of their structural, magnetic, biocompatibility and antibacterial properties. Mater Sci Eng, B 268:115119
Montazeran AH, Saber Samandari S, Khandan A (2018) Artificial intelligence investigation of three silicates bioceramics-magnetite bio-nanocomposite: hyperthermia and biomedical applications. Nanomedicine Journal 5:163–171
Sung Y-M, Shin Y-K, Ryu J-J (2007) Preparation of hydroxyapatite/zirconia bioceramic nanocomposites for orthopaedic and dental prosthesis applications. Nanotechnology 18:065602
Es-saddik M, Laasri S, Taha M, Laghzizil A, Guidara A, Chaari K et al (2021) Effect of the surface chemistry on the stability and mechanical properties of the Zirconia-Hydroxyapatite bioceramic. Surfaces and Interfaces 23:100980
Firouzjaei LG, Mohammadi M, Darzi GN, Nikzad M, Kazemi S (2021) Synthesis, characterization, and in-vitro evaluation of piperine-loaded silica/hydroxyapatite mesoporous nanoparticles. Chem Pap 75:6465–6475
Aghaei H, Nourbakhsh AA, Karbasi S, JavadKalbasi R, Rafienia M, Nourbakhsh N et al (2014) Investigation on bioactivity and cytotoxicity of mesoporous nano-composite MCM-48/hydroxyapatite for ibuprofen drug delivery. Ceram Int 40:7355–7362
Song Z, Liu Y, Shi J, Ma T, Zhang Z, Ma H et al (2018) Hydroxyapatite/mesoporous silica coated gold nanorods with improved degradability as a multi-responsive drug delivery platform. Mater Sci Eng, C 83:90–98
Yamada S, Tagaya M (2017) Analytical investigation of hydration and protein adsorption structures on hydroxyapatite-based mesoporous silica particles. Mater Lett 209:441–445
Guo X, Xue M, Chen F, Guo Q, Zhou X, Lin H et al (2022) Local delivery and controlled release of miR-34a loaded in hydroxyapatite/mesoporous organosilica nanoparticles composite-coated implant wire to accelerate bone fracture healing. Biomaterials 280:121300
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I et al (2017) Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng, C Mater Biol Appl 78:1246–1262
Maji K, Dasgupta S, Pramanik K, Bissoyi A (2016) Preparation and evaluation of gelatin–-chitosan-nanobioglass 3D porous Scaffold for bone tissue engineering. Int J Biomater 2016:9825659
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21:2529–2543
Abdelaal OA, Darwish SM. Review of rapid prototyping techniques for tissue engineering scaffolds fabrication. In: Characterization and development of biosystems and biomaterials. Springer, Berlin, pp 33–54
Li Z, Xie M-B, Li Y, Ma Y, Li J-S, Dai F-Y (2016) Recent progress in tissue engineering and regenerative medicine. J Biomater Tissue Eng 6:755–766
Eltom A, Zhong G, Muhammad A (2019) Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng 2019:1–13
Jahed E, Khaledabad MA, Almasi H, Hasanzadeh R (2017) Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohyd Polym 164:325–338
Hutmacher DW, Sittinger M, Risbud MV (2004) Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol 22:354–362
Maia FR, Bastos AR, Oliveira JM, Correlo VM, Reis RL (2022) Recent approaches towards bone tissue engineering. Bone 154:116256
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785
Kim G, Son J, Park S, Kim W (2008) Hybrid process for fabricating 3D hierarchical scaffolds combining rapid prototyping and electrospinning. Macromol Rapid Commun 29:1577–1581
Yu Y, Hua S, Yang M, Fu Z, Teng S, Niu K et al (2016) Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv 6:110557–110565
Vyas C, Ates G, Aslan E, Hart J, Huang B, Bartolo P (2020) Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print Add Manuf 7:105–113
Tortora GJ, Petti K (2002) Principles of human anatomy. Wiley, New York
Nair AK, Gautieri A, Chang S-W, Buehler MJ (2013) Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun 4:1724
Lopes D, Martins-Cruz C, Oliveira MB, Mano JF (2018) Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 185:240–275
Daculsi G, Fellah B, Miramond T, Durand M (2013) Osteoconduction, osteogenicity, osteoinduction, what are the fundamental properties for a smart bone substitutes. IRBM 34:346–348
Wu R, Li Y, Shen M, Yang X, Zhang L, Ke X et al (2021) Bone tissue regeneration: The role of finely tuned pore architecture of bioactive scaffolds before clinical translation. Bioact Mater 6:1242–1254
Dellavia C, Canciani E, Pellegrini G, Tommasato G, Graziano D, Chiapasco M (2021) Histological assessment of mandibular bone tissue after guided bone regeneration with customized computer-aided design/computer-assisted manufacture titanium mesh in humans: a cohort study. Clin Implant Dent Relat Res 23:600–611
Omar O, Engstrand T, Kihlstrom Burenstam Linder L, Aberg J, Shah FA, Palmquist A et al (2020) In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design. Proc Natl Acad Sci USA 117:26660–26671
Ghassemi T, Shahroodi A, Ebrahimzadeh MH, Mousavian A, Movaffagh J, Moradi A (2018) Current concepts in scaffolding for bone tissue engineering. Arch Bone Joint Surg 6:90
Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ (2005) Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res 20:848–857
Kolan KC, Huang Y-W, Semon JA, Leu MC (2020) 3D-printed biomimetic bioactive glass scaffolds for bone regeneration in rat calvarial defects. Int J Bioprint 6(2):82–98
Bozo IY, Deev RV, Smirnov IV, Fedotov AY, Popov VK, Mironov AV et al (2020) 3D printed gene-activated octacalcium phosphate implants for large bone defects engineering. Int J Bioprint 6(3):93–109
Kim W, Lee H, Ji Roh E, Bae An S, Han I-B, Hyung KG (2022) A multicellular bioprinted cell construct for vascularized bone tissue regeneration. Chem Eng J 431:133882
Yang L, Zhao Y, Cui D, Liu Y, Zou Q, Xu S et al (2022) Coaxial bioelectrospinning of P34HB/PVA microfibers biomimetic scaffolds with simultaneity cell-laden for improving bone regeneration. Mater Des 213:110349
Zhou Y, Liu X, She H, Wang R, Bai F, Xiang B (2022) A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects. Regen Ther 21:307–321
Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM (2005) Advancing cartilage tissue engineering: the application of stem cell technology. Curr Opin Biotechnol 16:503–509
Wang KY, Jin XY, Ma YH, Cai WJ, Xiao WY, Li ZW et al (2021) Injectable stress relaxation gelatin-based hydrogels with positive surface charge for adsorption of aggrecan and facile cartilage tissue regeneration. J Nanobiotechnol 19:214
Li Y, Xun X, Xu Y, Zhan A, Gao E, Yu F et al (2022) Hierarchical porous bacterial cellulose scaffolds with natural biomimetic nanofibrous structure and a cartilage tissue-specific microenvironment for cartilage regeneration and repair. Carbohyd Polym 276:118790
Haghighi P, Shamloo A (2021) Fabrication of a novel 3D scaffold for cartilage tissue repair: In-vitro and in-vivo study. Mater Sci Eng, C Mater Biol Appl 128:112285
Zhang Y, Guo W, Wang M, Hao C, Lu L, Gao S et al (2018) Co-culture systems-based strategies for articular cartilage tissue engineering. J Cell Physiol 233:1940–1951
Li W-J, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ et al (2005) A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26:599–609
Alves da Silva ML, Martins A, Costa-Pinto AR, Costa P, Faria S, Gomes M et al (2010) Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromol 11:3228–3236
Heirani-Tabasi A, Hosseinzadeh S, Rabbani S, Tafti SHA, Jamshidi K, Soufizomorrod M et al (2021) Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan–hyaluronic acid hydrogel. Biomed Mater 16:055003
Rathan S, Dejob L, Schipani R, Haffner B, Möbius ME, Kelly DJ (2019) Fiber reinforced cartilage ECM functionalized bioinks for functional cartilage tissue engineering. Adv Healthcare Mater 8:1801501
Winter GD (1963) Effect of air exposure and occlusion on experimental human skin wounds. Nature 200:378–379
Dhivya S, Padma VV, Santhini E (2015) Wound dressings—a review. Biomedicine 5:1–5
Geng X, Xu Z-Q, Tu C-Z, Peng J, Jin X, Ye L et al (2021) Hydrogel complex electrospun scaffolds and their multiple functions in in situ vascular tissue engineering. ACS Appl Bio Mater 4:2373–2384
Sun L, Li J, Gao W, Shi M, Tang F, Fu X et al (2021) Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. J Mater Chem B 9:1395–1405
Parvez S, Rahman MM, Khan MA, Khan MAH, Islam JMM, Ahmed M et al (2012) Preparation and characterization of artificial skin using chitosan and gelatin composites for potential biomedical application. Polym Bull 69:715–731
Sarkar SD, Farrugia BL, Dargaville TR, Dhara S (2013) Chitosan–collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. J Biomed Mater Res, Part A 101:3482–3492
Negut I, Grumezescu V, Grumezescu AM (2018) Treatment strategies for infected wounds. Molecules 23:2392
Maged A, Abdelkhalek AA, Mahmoud AA, Salah S, Ammar MM, Ghorab MM (2019) Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur J Pharm Sci 127:185–198
Sahoo S, Ang LT, Goh JCH, Toh SL (2010) Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res Part A 93:1539–1550
Zandi N, Dolatyar B, Lotfi R, Shallageh Y, Shokrgozar MA, Tamjid E et al (2021) Biomimetic nanoengineered scaffold for enhanced full-thickness cutaneous wound healing. Acta Biomater 124:191–204
Isseroff RR, Dahle SE (2012) Electrical Stimulation Therapy and Wound Healing: Where Are We Now? Adv Wound Care 1:238–243
Fu X, Wang JK, Ramírez-Pérez AC, Choong C, Lisak G (2020) Flexible conducting polymer-based cellulose substrates for on-skin applications. Mater Sci Eng, C 108:110392
Aycan D, Selmi B, Kelel E, Yildirim T, Alemdar N (2019) Conductive polymeric film loaded with ibuprofen as a wound dressing material. Eur Polymer J 121:109308
Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T et al (2016) Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE 11:e0158674
Ghahremanzadeh F, Alihosseini F, Semnani D (2021) Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int J Biol Macromol 174:278–288
Kaur S, Tripathi DM, Venugopal JR, Ramakrishna S (2020) Advances in biomaterials for hepatic tissue engineering. Curr Opin Biomed Engineering 13:190–196
Sk MM, Das P, Panwar A, Tan LP (2021) Synthesis and characterization of site selective photo-crosslinkable glycidyl methacrylate functionalized gelatin-based 3D hydrogel scaffold for liver tissue engineering. Mater Sci Eng, C Mater Biol Appl 123:111694
Li M, Khadim RR, Nagayama M, Shinohara M, Inamura K, Danoy M et al (2021) Fabrication of a porous three-dimensional scaffold with interconnected flow channels: co-cultured liver cells and in vitro hemocompatibility assessment. Appl Sci 11:2473
Xia L, Arooz T, Zhang S, Tuo X, Xiao G, Susanto TAK et al (2012) Hepatocyte function within a stacked double sandwich culture plate cylindrical bioreactor for bioartificial liver system. Biomaterials 33:7925–7932
Shang Y, Tamai M, Ishii R, Nagaoka N, Yoshida Y, Ogasawara M et al (2014) Hybrid sponge comprised of galactosylated chitosan and hyaluronic acid mediates the co-culture of hepatocytes and endothelial cells. J Biosci Bioeng 117:99–106
Lau TT, Ho LW, Wang D-A (2013) Hepatogenesis of murine induced pluripotent stem cells in 3D micro-cavitary hydrogel system for liver regeneration. Biomaterials 34:6659–6669
Brown JH, Das P, DiVito MD, Ivancic D, Tan LP, Wertheim JA (2018) Nanofibrous PLGA electrospun scaffolds modified with type I collagen influence hepatocyte function and support viability in vitro. Acta Biomater 73:217–227
Catto V, Farè S, Freddi G, Tanzi MC (2014) Vascular tissue engineering: recent advances in small diameter blood vessel regeneration. ISRN Vasc Med 2014:1–27
Dean EW, Udelsman B, Breuer CK (2012) Current advances in the translation of vascular tissue engineering to the treatment of pediatric congenital heart disease. Yale J Biol Med 85:229–238
Jiang C, Wang K, Liu Y, Zhang C, Wang B (2021) using wet electrospun PCL/Gelatin/CNT yarns to fabricate textile-based scaffolds for vascular tissue engineering. ACS Biomater Sci Eng 7:2627–2637
Dimopoulos A, Markatos DN, Mitropoulou A, Panagiotopoulos I, Koletsis E, Mavrilas D (2021) A novel polymeric fibrous microstructured biodegradable small-caliber tubular scaffold for cardiovascular tissue engineering. J Mater Sci Mater Med 32:21
Jun I, Han HS, Edwards JR, Jeon H (2018) Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci 19:745
Nikolova MP, Chavali MS (2019) Recent advances in biomaterials for 3D scaffolds: a review. Bioactive Mater 4:271–292
Spicer CD (2020) Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym Chem 11:184–219
Vijayavenkataraman S, Lu WF, Fuh JY (2016) 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 8:032001
Bishop ES, Mostafa S, Pakvasa M, Luu HH, Lee MJ, Wolf JM et al (2017) 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Diseases 4:185–195
Yu J, Park SA, Kim WD, Ha T, Xin Y-Z, Lee J et al (2020) Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers 12:2958
Wu C, Wang B, Zhang C, Wysk RA, Chen YW (2017) Bioprinting: an assessment based on manufacturing readiness levels. Crit Rev Biotechnol 37:333–354
Rodríguez-Salvador M, Rio-Belver RM, Garechana-Anacabe G (2017) Scientometric and patentometric analyses to determine the knowledge landscape in innovative technologies: the case of 3D bioprinting. PLoS ONE 12:e0180375
Forrestal DP, Klein TJ, Woodruff MA (2017) Challenges in engineering large customized bone constructs. Biotechnol Bioeng 114:1129–1139
Levato R, Visser J, Planell JA, Engel E, Malda J, Mateos-Timoneda MA (2014) Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 6:035020
Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly DJ (2016) 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater 5:2353–2362
Yang J, Zhang YS, Yue K, Khademhosseini A (2017) Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater 57:1–25
Ma X, Qu X, Zhu W, Li YS, Yuan S, Zhang H et al (2016) Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci USA 113:2206–2211
Kim Y, Kang K, Jeong J, Paik SS, Kim JS, Park SA et al (2017) Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure. Ann Surg Treat Res 92:67–72
Madariaga MLL, Ott HC (2014) Bioengineering kidneys for transplantation. In: Seminars in nephrology. Elsevier, Amsterdam, pp 384–393
Reint G, Rak-Raszewska A, Vainio SJ (2017) Kidney development and perspectives for organ engineering. Cell Tissue Res 369:171–183
Duan B (2017) State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng 45:195–209
Acknowledgements
The author would like to thank National Research Centre for support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Author information
Authors and Affiliations
Contributions
The author wrote the whole review independently.
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to disclose.
Ethics approval and consent to participate
The references of all the human participants, human data or human tissue were cited in this review.
Consent for publication
Not applicable.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, M.M. Recent trends on biomaterials for tissue regeneration applications: review. J Mater Sci 58, 527–558 (2023). https://doi.org/10.1007/s10853-022-08102-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-08102-x