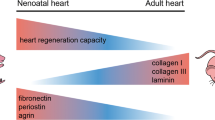
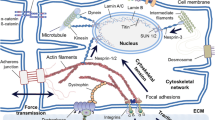
Abstract
Recent pre-clinical and clinical studies indicate that certain exogenous stem cells and biomaterials can preserve cardiac tissue after myocardial infarction. Regarding stem cells, a growing body of data suggests that the short-term positive outcomes are mainly attributed to paracrine signaling mechanisms. The release of such factors is due to the cell’s ability to sense cardiac environmentally derived cues, though the exact feedback loops are still poorly understood. However, given the limited engraftment and survival of transplanted cells in the ischemic environment, the long-term clinical benefits of these therapies have not yet been realized. To overcome this, the long-term controlled delivery of bioactive factors using biomaterials is a promising approach. A major challenge has been the ability to develop timely and spatially controlled gradients of different cues, pivotal for the development and regeneration of tissues. In addition, given the complexity of the remodeling process after myocardial infarction, multiple factors may be required at distinct disease stages to maximize therapeutic outcomes. Therefore, novel smart materials that can sense the surrounding environment and generate cues through on demand mechanisms will be of major importance in the translation of these promising advanced therapies. This article reviews how the cardiac environment can mediate the release profiles of bioactive cues from cells and biomaterials and how the controlled delivery impacts heart regeneration.



Similar content being viewed by others
References
Bergmann, O., Bhardwaj, R. D., Bernard, S., Zdunek, S., Barnabe-Heider, F., Walsh, S., et al. (2009). Evidence for cardiomyocyte renewal in humans. Science, 324(5923), 98–102. doi:10.1126/science.1164680.
Jessup, M., & Brozena, S. (2003). Medical progress: Heart failure. The New England Journal of Medicine, 348(20), 2007–2018. doi:10.1056/NEJMra021498.
Lloyd-Jones, D., Adams, R. J., Brown, T. M., Carnethon, M., Dai, S., De Simone, G., et al. (2010). Executive summary: Heart Disease and Stroke Statistics-2010 Update. A report from the American Heart Association. Circulation, 121(7), 948–954. doi:10.1161/circulationaha.109.192666.
Zimmermann, W. H. (2009). Remuscularizing failing hearts with tissue engineered myocardium. Antioxidants & Redox Signaling, 11(8), 2011–2023. doi:10.1089/ars.2009.2467.
Laflamme, M. A., & Murry, C. E. (2005). Regenerating the heart. Nature Biotechnology, 23(7), 845–856. doi:10.1038/nbt1117.
Segers, V. F. M., & Lee, R. T. (2010). Protein therapeutics for cardiac regeneration after myocardial infarction. Journal of Cardiovascular Translational Research, 3(5), 469–477. doi:10.1007/s12265-010-9207-5.
Dimmeler, S., Zeiher, A. M., & Schneider, M. D. (2005). Unchain my heart: The scientific foundations of cardiac repair. The Journal of Clinical Investigation, 115(3), 572–583. doi:10.1172/jci200524283.
Alexander, J. M., & Bruneau, B. G. (2010). Lessons for cardiac regeneration and repair through development. Trends in Molecular Medicine, 16(9), 426–434. doi:10.1016/j.molmed.2010.06.003.
Vandervelde, S., van Luyn, M. J. A., Tio, R. A., & Harmsen, M. C. (2005). Signaling factors in stem cell-mediated repair of infarcted myocardium. Journal of Molecular and Cellular Cardiology, 39(2), 363–376. doi:10.1016/j.yjmcc.2005.05.012.
Eaton, L. W., Weiss, J. L., Bulkley, B. H., Garrison, J. B., & Weisfeldt, M. L. (1979). Regional cardiac dilatation after acute myocardial-infarction—recognitior by 2-dimensional echocardiography. The New England Journal of Medicine, 300(2), 57–62. doi:10.1056/NEJM197905173002014.
Jugdutt, B. I. (2003). Ventricular remodeling after infarction and the extracellular collagen matrix—When is enough enough? Circulation, 108(11), 1395–1403. doi:10.1161/01.cir.0000085658.98621.49.
Ingber, D. E. (2002). Mechanical signalling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. CircRes, 91(10), 877–887. doi:10.1161/01.res.0000039537.73816.e5.
Dobaczewski, M., Gonzalez-Quesada, C., & Frangogiannis, N. G. (2009). The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. Journal of Molecular and Cellular Cardiology, 48(3), 504–511. doi:10.1016/j.yjmcc.2009.07.015.
Peterson, J. T., Li, H., Dillon, L., & Bryant, J. W. (2000). Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovascular Research, 46(2), 307–315. doi:10.1016/S0008-6363(00)00029-8.
Frangogiannis, N. G., Smith, C. W., & Entman, M. L. (2002). The inflammatory response in myocardial infarction. Cardiovascular Research, 53(1), 31–47. doi:10.1016/S0008-6363(01)00434-5.
Yellon, D. M., & Hausenloy, D. J. (2007). Mechanisms of disease: Myocardial reperfusion injury. The New England Journal of Medicine, 357(11), 1121–1135. doi:10.1056/NEJMra071667.
Murry, C. E., Jennings, R. B., & Reimer, K. A. (1986). Preconditioning with ischemia—a delay of lethal cell injury in ischemic myocardium. Circulation, 74(5), 1124–1136.
Lebuffe, G., Schumacker, P. T., Shao, Z. H., Anderson, T., Iwase, H., & Vanden Hoek, T. L. (2003). ROS and NO trigger early preconditioning: Relationship to mitochondrial K-ATP channel. Am J Physiol-Heart Circul Physiol, 284(1), H299–H308. doi:10.1152/ajpheart.00706.2002.
Garlid, K. D., Dos Santos, P., Xie, Z. J., Costa, A. D. T., & Paucek, P. (2003). Mitochondrial potassium transport: The role of the mitochondrial ATP-sensitive K + channel in cardiac function and cardioprotection. Biochim Biophys Acta-Bioenerg, 1606(1–3), 1–21. doi:10.1016/s0005-2728(03)00109-9.
Mukherjee, R., Mingoia, J. T., Bruce, J. A., Austin, J. S., Stroud, R. E., Escobar, G. P., et al. (2006). Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol-Heart Circul Physiol, 291(5), H2216–H2228. doi:10.1152/ajpheart.01343.2005.
Vanhoutte, D., Schellings, M., Pinto, Y., & Heymans, S. (2006). Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: A temporal and spatial window. Cardiovascular Research, 69(3), 604–613. doi:10.1016/j.cardiores.2005.10.002.
Webb, C. S., Bonnema, D. D., Ahmed, S. H., Leonardi, A. H., McClure, C. D., Clark, L. L., et al. (2006). Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction—Relation to left ventricular remodeling. Circulation, 114(10), 1020–1027. doi:10.1161/circulationaha.105.600353.
Matsumura, S., Iwanaga, S., Mochizuki, S., Okamoto, H., Ogawa, S., & Okada, Y. (2005). Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. The Journal of Clinical Investigation, 115(3), 599–609. doi:10.1172/jci200522304.
Olivetti, G., Capasso, J. M., Sonnenblick, E. H., & Anversa, P. (1990). Side-to-side slippage of myocites participated in ventricular wall remodelling acutely after myocardial-infarction rats. CircRes, 67(1), 23–34.
Askari, A. T., Unzek, S., Popovic, Z. B., Goldman, C. K., Forudi, F., Kiedrowski, M., et al. (2003). Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet, 362(9385), 697–703. doi:10.1016/S0140-6736(03)14232-8.
Abbott, J. D., Huang, Y., Liu, D., Hickey, R., Krause, D. S., & Giordano, F. J. (2004). Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation, 110(21), 3300–3305. doi:10.1161/01.cir.0000147780.30124.cf.
Kucia, M., Dawn, B., Hunt, G., Guo, Y. R., Wysoczynski, M., Majka, M., et al. (2004). Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. CircRes, 95(12), 1191–1199. doi:10.1161/01.RES.0000150856.47324.5b.
Ankrum, J., & Karp, J. M. (2010). Mesenchymal stem cell therapy: Two steps forward, one step back. Trends in Molecular Medicine, 16(5), 203–209. doi:10.1016/j.molmed.2010.02.005.
Yang, Z. Q., Zingarelli, B., & Szabo, C. (2000). Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation, 101(9), 1019–1026.
Burchfield, J. S., Iwasaki, M., Koyanagi, M., Urbich, C., Rosenthal, N., Zeiher, A. M., et al. (2008). Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. CircRes, 103(2), 203–211. doi:10.1161/circresaha.108.178475.
Tziakas, D. N., Chalikias, G. K., Hatzinikolaou, H. I., Parissis, J. T., Papadopoulos, E. D., Trypsianis, G. A., et al. (2003). Anti-inflammatory cytokine profile in acute coronary syndromes: Behavior of interleukin-10 in association with serum metalloproteinases and proinflammatory cytokines. International Journal of Cardiology, 92(2–3), 169–175. doi:10.1016/s0167-5273(03)00084-6.
Krishnamurthy, P., Lambers, E., Verma, S., Thorne, T., Qin, G. J., Losordo, D. W., et al. (2010). Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. The FASEB Journal, 24(7), 2484–2494. doi:10.1096/fj.09-149815.
Bujak, M., & Frangogiannis, N. G. (2007). The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovascular Research, 74(2), 184–195. doi:10.1016/j.cardiores.2006.10.002.
Dewald, O., Zymek, P., Winkelmann, K., Koerting, A., Ren, G. F., Abou-Khamis, T., et al. (2005). CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. CircRes, 96(8), 881–889. doi:10.1161/01.RES.0000163017.13772.3a.
Eghbali, M., Tomek, R., Woods, C., & Bhambi, B. (1991). Cardiac fibroblast are predisposed to convert into myocyte phenotype—specific effect of transforming growth-factor-beta. Proceedings of the National Academy of Sciences of the United States of America, 88(3), 795–799. doi:10.1073/pnas.88.3.795.
Cleutjens, J. P. M., Verluyten, M. J. A., Smits, J. F. M., & Daemen, M. (1995). Collagen remodeling after myocardial-infarction in the rat-heart. Am J Pathol, 147(2), 325–338.
van den Borne, S. W. M., Diez, J., Blankesteijn, W. M., Verjans, J., Hofstra, L., & Narula, J. (2009). Myocardial remodeling after infarction: The role of myofibroblasts. Nat Rev Cardiol, 7(1), 30–37. doi:10.1038/nrcardio.2009.199.
McCormick, R. J., Musch, T. I., Bergman, B. C., & Thomas, D. P. (1994). Regional differences in LV collagen accumulation and mature cross-linking after myocardial-infarction in rats. Am J Physiol, 266(1), H354–H359.
Gnecchi, M., Zhang, Z. P., Ni, A. G., & Dzau, V. J. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research, 103(11), 1204–1219. doi:10.1161/circresaha.108.176826.
Hwang, H., & Kloner, R. A. (2010). Improving regenerating potential of the heart after myocardial infarction: Factor-based approach. Life Sciences, 86(13–14), 461–472. doi:10.1016/j.lfs.2010.01.004.
Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297. doi:10.1016/S0092-8674(04)00045-5.
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S. K., Choo, A., Chen, T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4(3), 214–222. doi:10.1016/j.scr.2009.12.003.
Chen, T. S., Lai, R. C., Lee, M. M., Choo, A. B. H., Lee, C. N., & Lim, S. K. (2010). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research, 38(1), 215–224. doi:10.1093/nar/gkp857.
Hu SJ, Huang M, Li ZJ, Jia FJ, Ghosh ZM, Lijkwan MA, Fasanaro P, Sun N, Wang X, Li FM, Robbins RC, Wu JC MicroRNA-210 as a Novel Therapy for Treatment of Ischemic Heart Disease. Circulation 122 (11):S124-S131. doi:10.1161/circulationaha.109.928424
van der Bogt, K. E. A., Sheikh, A. Y., Schrepfer, S., Hoyt, G., Cao, F., Ransohoff, K. J., et al. (2008). Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation, 118(14), S121–U166. doi:10.1161/circulationaha.107.759480.
Arminan, A., Gandia, C., Garcia-Verdugo, J. M., Lledo, E., Trigueros, C., Ruiz-Sauri, A., et al. (2009). Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. Journal of the American College of Cardiology, 55(20), 2244–2253. doi:10.1016/j.jacc.2009.08.092.
Kamihata, H., Matsubara, H., Nishiue, T., Fujiyama, S., Tsutsumi, Y., Ozono, R., et al. (2001). Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation, 104(9), 1046–1052. doi:10.1161/hc3501.093817.
Kraehenbuehl, T. P., Ferreira, L. S., Hayward, A. M., Nahrendorf, M., van der Vlies, A. J., Vasile, E., et al. (2010). Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials, 32(4), 1102–1109. doi:10.1016/j.biomaterials.2010.10.005.
Chimenti, I., Smith, R. R., Li, T. S., Gerstenblith, G., Messina, E., Giacomello, A., et al. (2010). Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. CircRes, 106(5), 971–U304. doi:10.1161/circresaha.109.210682.
Suzuki K, Murtuza B, Beauchamp JR, Brand NJ, Barton PJR, Varela-Carver A, Fukushima S, Coppen SR, Partridge TA, Yacoub MH (2004) Role of interleukin-1 beta in acute inflammation and graft death after cell transplantation to the heart. Circulation 110 (11):II219-II224. doi:10.1161/01.cir.0000138388.55416.06
Shintani, Y., Fukushima, S., Varela-Carver, A., Lee, J., Coppen, S. R., Takahashi, K., et al. (2009). Donor cell-type specific paracrine effects of cell transplantation for post-infarction heart failure. Journal of Molecular and Cellular Cardiology, 47(2), 288–295. doi:10.1016/j.yjmcc.2009.05.009.
Mirotsou, M., Zhang, Z. Y., Deb, A., Zhang, L. N., Gnecchi, M., Noiseux, N., et al. (2007). Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proceedings of the National Academy of Sciences of the United States of America, 104(5), 1643–1648. doi:10.1073/pnas.0610024104.
Wang, M. J., Tan, J. N., Wang, Y., Meldrum, K. K., Dinarello, C. A., & Meldrum, D. R. (2009). IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proceedings of the National Academy of Sciences of the United States of America, 106(41), 17499–17504. doi:10.1073/pnas.0908924106.
Li, W. Z., Ma, N., Ong, L. L., Nesselmann, C., Klopsch, C., Ladilov, Y., et al. (2007). Bc1-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells, 25(8), 2118–2127. doi:10.1634/stemcells.2006-0771.
Wang, X. H., Zhao, T. M., Huang, W., Wang, T., Qian, J., Xu, M. F., et al. (2009). Hsp20-Engineered mesenchymal stem cells Are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells, 27(12), 3021–3031. doi:10.1002/stem.230.
Huang, J., Zhang, Z. P., Guo, J. A., Ni, A. G., Deb, A., Zhang, L. N., et al. (2010). Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. CircRes, 106(11), 1753–U1189. doi:10.1161/circresaha.109.196030.
Pouyssegur, J., Dayan, F., & Mazure, N. M. (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441(7092), 437–443. doi:10.1038/nature04871.
Chen, L. W., Tredget, E. E., Wu, P. Y. G., & Wu, Y. J. (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One, 3(4), 12. doi:10.1371/journal.pone.0001886, e1886.
Wang, M. J., Crisostomo, P. R., Herring, C., Meldrum, K. K., & Meldrum, D. R. (2006). Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291(4), R880–R884. doi:10.1152/ajpregu.00280.2006.
Kelly, M. L., Wang, M. J., Crisostomo, P. R., Abarbanell, A. M., Herrmann, J. L., Weil, B. R., et al. (2009). TNF recepter 2, not TNF receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock, 33(6), 602–607. doi:10.1097/SHK.0b013e3181cc0913.
Bao, C. Y., Guo, J., Zheng, M., Chen, Y., Lin, G. S., & Hu, M. Y. (2010). Enhancement of the survival of engrafted mesenchymal stem cells in the ischemic heart by TNFR gene transfection. Biochemistry and Cell Biology, 88(4), 629–634. doi:10.1139/010-018.
Chang, S. A., Lee, E. J., Kang, H. J., Zhang, S. Y., Kim, J. H., Li, L., et al. (2008). Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: Effect of acute myocardial infarction on stem cell differentiation. Stem Cells, 26(7), 1901–1912. doi:10.1634/stemcells.2007-0708.
Lee, R. H., Pulin, A. A., Seo, M. J., Kota, D. J., Ylostalo, J., Larson, B. L., et al. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 5(1), 54–63. doi:10.1016/j.stem.2009.05.003.
Nauta, A. J., & Fibbe, W. E. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood, 110(10), 3499–3506. doi:10.1182/blood-2007-02-069716.
Meirelles, L. D., Fontes, A. M., Covas, D. T., & Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews, 20(5–6), 419–427. doi:10.1016/j.cytogfr.2009.10.002.
Laterveer, L., Lindley, I. J. D., Hamilton, M. S., Willemze, R., & Fibbe, W. E. (1995). Interleukin-8 induces rapid mobilization of hematopoietic stem-cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood, 85(8), 2269–2275.
Bocker, W. G., Docheva, D., Prall, W. C., Egea, V., Pappou, E., Rossmann, O., et al. (2008). IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. Journal of Molecular Medicine, 86(10), 1183–1192. doi:10.1007/s00109-008-0378-3.
Asahara, T., Takahashi, T., Masuda, H., Kalka, C., Chen, D. H., Iwaguro, H., et al. (1999). VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J, 18(14), 3964–3972.
Tang, Y. L., Zhu, W. Q., Cheng, M., Chen, L. J., Zhang, J., Sun, T., et al. (2009). Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. CircRes, 104(10), 1209–U1218. doi:10.1161/circresaha.109.197723.
Nakamuta, J. S., Danoviz, M. E., Marques, F. L. N., dos Santos, L., Becker, C., Goncalves, G. A., et al. (2009). Cell therapy attenuates cardiac dysfunction post myocardial infarction: Effect of timing, routes of injection and a fibrin scaffold. PloS One, 4(6), 10. doi:10.1371/journal.pone.0006005, e6005.
Barbash, I. M., Chouraqui, P., Baron, J., Feinberg, M. S., Etzion, S., Tessone, A., et al. (2003). Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium—Feasibility, cell migration, and body distribution. Circulation, 108(7), 863–868. doi:10.1161/01.cir.0000084828.50310.6a.
Karp, J. M., & Teol, G. S. L. (2009). Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell, 4(3), 206–216. doi:10.1016/j.stem.2009.02.001.
Manning, M. C., Chou, D. K., Murphy, B. M., Payne, R. W., & Katayama, D. S. (2010). Stability of protein pharmaceuticals: An update. Pharmaceutical Research, 27(4), 544–575. doi:10.1007/s11095-009-0045-6.
Davis, M. E., Hsieh, P. C. H., Grodzinsky, A. J., & Lee, R. T. (2005). Custom design of the cardiac microenvironment with biomaterials. CircRes, 97(1), 8–15. doi:10.1161/01.res.0000173376.39447.01.
Ferreira, L., Pedroso, D. C., Vazão, H., & Gomes, R. S. (2010). Stem cell-based therapies for heart regeneration: What did the bench teach us? Cardiovascular & Hematological Disorders Drug Targets, 10(3), 173–185.
Freeman, I., & Cohen, S. (2009). The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials, 30(11), 2122–2131. doi:10.1016/j.biomaterials.2008.12.057.
Chen, F. M., Zhang, M., & Wu, Z. F. (2010). Toward delivery of multiple growth factors in tissue engineering. Biomaterials, 31(24), 6279–6308. doi:10.1016/j.biomaterials.2010.04.053.
Mukherjee, S., Venugopal, J. R., Ravichandran, R., Ramakrishna, S., & Raghunath, M. (2010). Multimodal biomaterial strategies for regeneration of infarcted myocardium. Journal of Materials Chemistry, 20(40), 8819–8831. doi:10.1039/c0jm00805b.
Lindahl U, Li JP (2009) Interactions between heparan sulfate and proteins-design and functional implications. In: International Review of Cell and Molecular Biology, Vol 276, vol 276. International Review of Cell and Molecular Biology. Elsevier Academic Press Inc, San Diego, pp 105–159. doi:10.1016/s1937-6448(09)76003-4
Kraehenbuehl, T. P., Ferreira, L. S., Zammaretti, P., Hubbell, J. A., & Langer, R. (2009). Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials, 30(26), 4318–4324. doi:10.1016/j.biomaterials.2009.04.057.
Vazao, H., das Neves, R. P., Graos, M., & Ferreira, L. (2011). Towards the maturation and characterization of smooth muscle cells derived from human embryonic stem cells. PloS One, 6(3), 14. doi:10.1371/journal.pone.0017771.
Ferreira, L. S., Gerecht, S., Shieh, H. F., Watson, N., Rupnick, M. A., Dallabrida, S. M., et al. (2007). Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle-like cells and form vascular networks in vivo. CircRes, 101(3), 286–294. doi:10.1161/circresaha.107.150201.
Kraehenbuehl, T. P., Zammaretti, P., Van der Vlies, A. J., Schoenmakers, R. G., Lutolf, M. P., Jaconi, M. E., et al. (2008). Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials, 29(18), 2757–2766. doi:10.1016/j.biomaterials.2008.03.016.
Sladek, T., Filkuka, J., Dolezel, S., Vasku, J., Hartmannova, B., & Travnickova, J. (1984). The border zone of the early myocardial-infarction in dogs—its characteristics and viability. Basic Research in Cardiology, 79(3), 344–349. doi:10.1007/BF01908035.
Sy, J. C., Seshadri, G., Yang, S. C., Brown, M., Oh, T., Dikalov, S., et al. (2008). Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nature Materials, 7(11), 863–869. doi:10.1038/nmat2299.
Gupta, P., Vermani, K., & Garg, S. (2002). Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discovery Today, 7(10), 569–579. doi:10.1016/S1359-6446(02)02255-9.
Qiu, Y., & Park, K. (2001). Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews, 53(3), 321–339. doi:10.1016/S0169-409X(01)00203-4.
Lowman, A. M., Morishita, M., Kajita, M., Nagai, T., & Peppas, N. A. (1999). Oral delivery of insulin using pH-responsive complexation gels. Journal of Pharmaceutical Sciences, 88(9), 933–937. doi:10.1021/js980337n.
Miyata, T., Uragami, T., & Nakamae, K. (2002). Biomolecule-sensitive hydrogels. Advanced Drug Delivery Reviews, 54(1), 79–98. doi:10.1016/S0169-409X(01)00241-1.
Miyata, T., Asami, N., & Uragami, T. (1999). A reversibly antigen-responsive hydrogel. Nature, 399(6738), 766–769. doi:10.1038/21619.
Matsumoto, A., Yoshida, R., & Kataoka, K. (2004). Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules, 5(3), 1038–1045. doi:10.1021/bm0345413.
Wilson DS, Dalmasso G, Wang LX, Sitaraman SV, Merlin D, Murthy N Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 9 (11):923–928. doi:10.1038/nmat2859
Broaders, K. E., Grandhe, S., & Frechet, J. M. J. (2011). A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. Journal of the American Chemical Society, 133(4), 756–758. doi:10.1021/ja110468v.
Lee, K. Y., Peters, M. C., Anderson, K. W., & Mooney, D. J. (2000). Controlled growth factor release from synthetic extracellular matrices. Nature, 408(6815), 998–1000. doi:10.1038/35050141.
Labhasetwar, V., Underwood, T., Schwendeman, S. P., & Levy, R. J. (1995). Iontophoresis for modulation of cardiac drug-delivery in dogs. Proceedings of the National Academy of Sciences of the United States of America, 92(7), 2612–2616.
Zhao, X. H., Kim, J., Cezar, C. A., Huebsch, N., Lee, K., Bouhadir, K., et al. (2010). Active scaffolds for on-demand drug and cell delivery. Proceedings of the National Academy of Sciences of the United States of America, 108(1), 67–72. doi:10.1073/pnas.1007862108.
Hausenloy, D. J., & Yellon, D. M. (2009). Cardioprotective growth factors. Cardiovascular Research, 83(2), 179–194. doi:10.1093/cvr/cvp062.
Kobayashi, H., Minatoguchi, S., Yasuda, S., Bao, N., Kawamura, I., Iwasa, M., et al. (2008). Post-infarct treatment with an erythropoietin-gelatin hydrogel drug delivery system for cardiac repair. Cardiovascular Research, 79(4), 611–620. doi:10.1093/cvr/cvn154.
Davis, M. E., Hsieh, P. C. H., Takahashi, T., Song, Q., Zhang, S. G., Kamm, R. D., et al. (2006). Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America, 103(21), 8155–8160. doi:10.1073/pnas.0602877103.
Hao, X. J., Silva, E. A., Mansson-Broberg, A., Grinnemo, K. H., Siddiqui, A. J., Dellgren, G., et al. (2007). Angiogenic effects of sequential release of VEGF-A(165) and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovascular Research, 75(1), 178–185. doi:10.1016/j.cardiores.2007.03.028.
Formiga, F. R., Pelacho, B., Garbayo, E., Abizanda, G., Gavira, J. J., Simon-Yarza, T., et al. (2010). Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia-reperfusion model. Journal of Controlled Release, 147(1), 30–37. doi:10.1016/j.jconrel.2010.07.097.
Seliktar, D., Zisch, A. H., Lutolf, M. P., Wrana, J. L., & Hubbell, J. A. (2004). MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. Journal of Biomedical Materials Research. Part A, 68A(4), 704–716. doi:10.1002/jbm.a.20091.
Silva, E. A., Kim, E. S., Kong, H. J., & Mooney, D. J. (2008). Material-based deployment enhances efficacy of endothelial progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 105(38), 14347–14352. doi:10.1073/pnas.0803873105.
Miyagi, Y., Chiu, L. L. Y., Cimini, M., Weisel, R. D., Radisic, M., & Li, R. K. (2010). Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials, 32(5), 1280–1290. doi:10.1016/j.biomaterials.2010.10.007.
Segers, V. F. M., Tokunou, T., Higgins, L. J., MacGillivray, C., Gannon, J., & Lee, R. T. (2007). Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation, 116(15), 1683–1692. doi:10.1161/circulationaha.107.718718.
Calvillo, L., Latini, R., Kajstura, J., Leri, A., Anversa, P., Ghezzi, P., et al. (2003). Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4802–4806. doi:10.1073/pnas.0630444100.
Wang, T., Jiang, X. J., Lin, T., Ren, S., Li, X. Y., Zhang, X. Z., et al. (2009). The inhibition of postinfarct ventricle remodeling without polycythaemia following local sustained intramyocardial delivery of erythropoietin within a supramolecular hydrogel. Biomaterials, 30(25), 4161–4167. doi:10.1016/j.biomaterials.2009.04.033.
Ruvinov E, Leor J, Cohen S The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 32 (2):565–578. doi:10.1016/j.biomaterials.2010.08.097
Fraidenraich, D., Stillwell, E., Romero, E., Wilkes, D., Manova, K., Basson, C. T., et al. (2004). Rescue of cardiac defects in Id knockout embryos by injection of embryonic stem cells. Science, 306(5694), 247–252.
Tokunou, T., Miller, R., Patwari, P., Davis, M. E., Segers, V. F. M., Grodzinsky, A. J., et al. (2008). Engineering insulin-like growth factor-1 for local delivery. The FASEB Journal, 22(6), 1886–1893. doi:10.1096/fj.07-100925.
Ota, T., Gilbert, T. W., Schwartzman, D., McTiernan, C. F., Kitajima, T., Ito, Y., et al. (2008). A fusion protein of hepatocyte growth factor enhances reconstruction of myocardium in a cardiac patch derived from porcine urinary bladder matrix. The Journal of Thoracic and Cardiovascular Surgery, 136(5), 1309–1317. doi:10.1016/j.jtcvs.2008.07.008.
Cao, Y. H., Hong, A., Schulten, H., & Post, M. J. (2005). Update on therapeutic neovascularization. Cardiovascular Research, 65(3), 639–648. doi:10.1016/j.cardiores.2004.11.020.
Sivakumar, B., Harry, L. E., & Paleolog, E. M. (2004). Modulating angiogenesis—More vs less. JAMA-J Am Med Assoc, 292(8), 972–977. doi:10.1001/jama.292.8.972.
Le, K., Hwang, C. W., Tzafriri, A. R., Lovich, M., & Edelman, E. (2008). Local therapeutic angiogenesis is limited by microvascular clearance. Circulation, 118(18), S457–S457.
Dor, Y., Djonov, V., & Keshet, E. (2003). Induction of vascular networks in adult organs: Implications to proangiogenic therapy. In M. NilsenHamilton, Z. Werb, & E. Keshet (Eds.), Tissue remodeling, vol 995. Annals of the New York Academy of Sciences (pp. 208–215). New York: New York Acad Sciences.
Dor, Y., Djonov, V., Abramovitch, R., Itin, A., Fishman, G. I., Carmeliet, P., et al. (2002). Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. Embo J, 21(8), 1939–1947.
Thurston, G., Suri, C., Smith, K., McClain, J., Sato, T. N., Yancopoulos, G. D., et al. (1999). Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science, 286(5449), 2511–2514. doi:10.1126/science.286.5449.2511.
Hariawala, M. D., Horowitz, J. R., Esakof, D., Sheriff, D. D., Walter, D., Keyt, B., et al. (1996). VEGF improves myocardial blood flow but produces EDRF-mediated hypotension in porcine hearts. J Surg Res, 63(1), 77–82. doi:10.1006/jsre.1996.0226.
Ozawa, C. R., Banfi, A., Glazer, N. L., Thurston, G., Springer, M. L., Kraft, P. E., et al. (2004). Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. The Journal of Clinical Investigation, 113(4), 516–527. doi:10.1172/jci200418420.
Lee, R. J., Springer, M. L., Blanco-Bose, W. E., Shaw, R., Ursell, P. C., & Blau, H. M. (2000). VEGF gene delivery to myocardium—Deleterious effects of unregulated expression. Circulation, 102(8), 898–901.
Henry, T. D., Annex, B. H., McKendall, G. R., Azrin, M. A., Lopez, J. J., Giordano, F. J., et al. (2003). Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation, 107(10), 1359–1365. doi:10.1161/01.cir.0000061911.47710.8a.
Laham, R. J., Sellke, F. W., Edelman, E. R., Pearlman, J. D., Ware, J. A., Brown, D. L., et al. (1999). Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery—Results of a phase I randomized, double-blind, placebo-controlled trial. Circulation, 100(18), 1865–1871.
Ruel, M., Laham, R. J., Parker, J. A., Post, M. J., Ware, J. A., Simons, M., et al. (2002). Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. The Journal of Thoracic and Cardiovascular Surgery, 124(1), 28–34. doi:10.1067/mtc.2002.121974.
Simons, M., Annex, B. H., Laham, R. J., Kleiman, N., Henry, T., Dauerman, H., et al. (2002). Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2—Double-blind, randomized, controlled clinical trial. Circulation, 105(7), 788–793. doi:10.1161/hc0802.104407.
Cao, R. H., Brakenhielm, E., Pawliuk, R., Wariaro, D., Post, M. J., Wahlberg, E., et al. (2003). Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nature Medicine, 9(5), 604–613. doi:10.1038/nm848.
Hsieh, P. C. H., Davis, M. E., Lisowski, L. K., & Lee, R. T. (2006). Endothelial-cardiomyocyte interactions in cardiac development and repair. Annual Review of Physiology, 68, 51–66. doi:10.1146/annurev.physiol.68.040104.124629.
Dvir, T., Kedem, A., Ruvinov, E., Levy, O., Freeman, I., Landa, N., et al. (2009). Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proceedings of the National Academy of Sciences of the United States of America, 106(35), 14990–14995. doi:10.1073/pnas.0812242106.
McQuibban, G. A., Butler, G. S., Gong, J. H., Bendall, L., Power, C., Clark-Lewis, I., et al. (2001). Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem, 276(47), 43503–43508. doi:10.1074/jbc.M107736200.
Zaruba, M. M., Theiss, H. D., Vallaster, M., Mehl, U., Brunner, S., David, R., et al. (2009). Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell, 4(4), 313–323. doi:10.1016/j.stem.2009.02.013.
Penn, M. S. (2009). Importance of the SDF-1:CXCR4 axis in myocardial repair. CircRes, 104(10), 1133–1135. doi:10.1161/circresaha.109.198929.
Petit, I., Szyper-Kravitz, M., Nagler, A., Lahav, M., Peled, A., Habler, L., et al. (2002). G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology, 3(7), 687–694. doi:10.1038/ni813.
Landa, N., Miller, L., Feinberg, M. S., Holbova, R., Shachar, M., Freeman, I., et al. (2008). Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation, 117(11), 1388–1396. doi:10.1161/circulationaha.107.727420.
Wall, S. T., Walker, J. C., Healy, K. E., Ratcliffe, M. B., & Guccione, J. M. (2006). Theoretical impact of the injection of material into the myocardium—A finite element model simulation. Circulation, 114(24), 2627–2635. doi:10.1161/circulationaha.106.657270.
Nelson, D. M., Ma, Z. W., Fujimoto, K. L., Hashizume, R., & Wagner, W. R. (2010). Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomaterialia, 7(1), 1–15. doi:10.1016/j.actbio.2010.06.039.
Stastna, M., Chimenti, I., Marban, E., & Van Eyk, J. E. (2009). Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics, 10(2), 245–253. doi:10.1002/pmic.200900515.
Siwik, D. A., Chang, D. L. F., & Colucci, W. S. (2000). Interleukin-1 beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. CircRes, 86(12), 1259–1265.
Finkel, M. S., Hoffman, R. A., Shen, L., Oddis, C. V., Simmons, R. L., & Hattler, B. G. (1993). Interleukin-6 (IL-6) as a mediator of stunned myocardium. The American Journal of Cardiology, 71(13), 1231–1232. doi:10.1016/0002-9149(93)90654-U.
Kukielka, G. L., Smith, C. W., Manning, A. M., Youker, K. A., Michael, L. H., & Entman, M. L. (1995). Induction of interleukin-6 synthesis in the myocardium—potential role is postreperfusion inflammatory injury. Circulation, 92(7), 1866–1875.
Lacraz, S., Nicod, L. P., Chicheportiche, R., Welgus, H. G., & Dayer, J. M. (1995). IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. The Journal of Clinical Investigation, 96(5), 2304–2310. doi:10.1172/JCI118286.
Frangogiannis, N. G., Mendoza, L. H., Lindsey, M. L., Ballantyne, C. M., Michael, L. H., Smith, C. W., et al. (2000). IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol, 165(5), 2798–2808.
Sun, M., Dawood, F., Wen, W. H., Chen, M. Y., Dixon, I., Kirshenbaum, L. A., et al. (2004). Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation, 110(20), 3221–3228. doi:10.1161/01.cir.0000147233.10318.23.
Yokoyama, T., Vaca, L., Rossen, R. D., Durante, W., Hazarika, P., & Mann, D. L. (1993). Cellular basis for the negative inotropic effects of tumor-necrosis-factor-alpha in the asult mammalian heart. The Journal of Clinical Investigation, 92(5), 2303–2312. doi:10.1172/JCI116834.
Frangogiannis, N. G., Lindsey, M. L., Michael, L. H., Youker, K. A., Bressler, R. B., Mendoza, L. H., et al. (1998). Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation, 98(7), 699–710.
Acknowledgments
This work was supported in part by a Marie Curie-Reintegration Grant (FP7-People-2007-4-3-IRG; contract no. 230929), the MIT-Portugal program, the Portuguese Foundation for Science and Technology (FCT; PTDC/SA-BEB/098468/2008; PTDC/CTM/099659/2008) and Crioestaminal (project no. CENTRO-01-0202-FEDER-005476 “INJECTCORD”) to LF. This work was also supported by the National Institute of Health grants DE019191, HL095722, and HL097172 to JMK. The authors would also like to thank FCT for the fellowship to MNP (SFRH/BD/43013/2008).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pereira, M.J.N., Carvalho, I.F., Karp, J.M. et al. Sensing the Cardiac Environment: Exploiting Cues for Regeneration. J. of Cardiovasc. Trans. Res. 4, 616–630 (2011). https://doi.org/10.1007/s12265-011-9299-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9299-6