Abstract
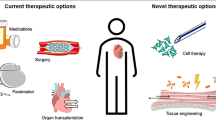
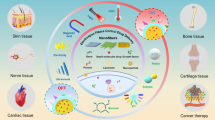
The spectrum of ischemic heart diseases, encompassing acute myocardial infarction to heart failure, represents the leading cause of death worldwide. Although extensive progress in cardiovascular diagnoses and therapy has been made, the prevalence of the disease continues to increase. Cardiac regeneration has a promising perspective for the therapy of heart failure. Recently, extracellular matrix (ECM) has been shown to play an important role in cardiac regeneration and repair after cardiac injury. There is also evidence that the ECM could be directly used as a drug to promote cardiomyocyte proliferation and cardiac regeneration. Increasing evidence supports that applying ECM biomaterials to maintain heart function recovery is an important approach to apply the concept of cardiac regenerative medicine to clinical practice in the future. Here, we will introduce the essential role of cardiac ECM in cardiac regeneration and summarize the approaches of delivering ECM biomaterials to promote cardiac repair in this review.


Similar content being viewed by others
References
Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K et al (2017) Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res 121(6):677–694. https://doi.org/10.1161/circresaha.117.308903
Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med 366(1):54–63. https://doi.org/10.1056/NEJMra1112570
Xin M, Olson EN, Bassel-Duby R (2013) Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol 14(8):529–541. https://doi.org/10.1038/nrm3619
Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O et al (2017) The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547(7662):179–184. https://doi.org/10.1038/nature22978
Eroglu E, Chien KR (2017) Heart regeneration 4.0: Matrix Medicine. Dev Cell 42(1):7–8. https://doi.org/10.1016/j.devcel.2017.06.017
Shiro Yui LA, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, Johansen JV, Li Y, Madsen CD, Nakamura T, Watanabe M, Nielsen OH, Schweiger PJ, Piccolo S, Jensen KB (2018) YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22(1):35–49 e7
Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA (2017) Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20(1):56–59. https://doi.org/10.1016/j.stem.2016.09.010
Kutys ML, Yamada KM (2014) An extracellular-matrix-specific GEF-GAP interaction regulates Rho GTPase crosstalk for 3D collagen migration. Nat Cell Biol 16(9):909–917. https://doi.org/10.1038/ncb3026
Rozario T, DeSimone DW (2010) The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 341(1):126–140. https://doi.org/10.1016/j.ydbio.2009.10.026
Frangogiannis NG (2017) The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127(5):1600–1612. https://doi.org/10.1172/jci87491
Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T et al (2017) The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev 121:43–56. https://doi.org/10.1016/j.addr.2017.07.014
Bashey RI, Martinez-Hernandez A, Jimenez SA (1992) Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circ Res 70(5):1006–1017
Jugdutt BI (2003) Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 108(11):1395–1403. https://doi.org/10.1161/01.cir.0000085658.98621.49
Astrof S, Kirby A, Lindblad-Toh K, Daly M, Hynes RO (2007) Heart development in fibronectin-null mice is governed by a genetic modifier on chromosome four. Mech Dev 124(7-8):551–558. https://doi.org/10.1016/j.mod.2007.05.004
Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A et al (2008) Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 102(7):752–760. https://doi.org/10.1161/circresaha.107.159517
Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD 3rd. (2014) Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther 5(1):14. https://doi.org/10.1186/scrt403
Chen WC, Wang Z, Missinato MA, Park DW, Long DW, Liu HJ et al (2016) Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci Adv 2(11):e1600844. https://doi.org/10.1126/sciadv.1600844
Mercer SE, Odelberg SJ, Simon HG (2013) A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev Biol 382(2):457–469. https://doi.org/10.1016/j.ydbio.2013.08.002
Lindsey ML (2018) Assigning matrix metalloproteinase roles in ischaemic cardiac remodelling. Nat Rev Cardiol 15(8):471–479. https://doi.org/10.1038/s41569-018-0022-z
Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG (2006) Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res 324(3):475–488. https://doi.org/10.1007/s00441-005-0144-6
Li L, Zhao Q, Kong W (2018) Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol 68-69:490–506. https://doi.org/10.1016/j.matbio.2018.01.013
Ren G, Michael LH, Entman ML, Frangogiannis NG (2002) Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 50(1):71–79. https://doi.org/10.1177/002215540205000108
Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L et al (2001) Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 88(10):1080–1087. https://doi.org/10.1161/hh1001.090842
Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE et al (2009) Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206(1):113–123. https://doi.org/10.1084/jem.20081244
Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A et al (2005) Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 111(22):2935–2942. https://doi.org/10.1161/circulationaha.104.510354
Brockes JP (1997) Amphibian limb regeneration: rebuilding a complex structure. Science (New York, NY) 276(5309):81–87
Poss KD, Wilson LG, Keating MT (2002) Heart regeneration in zebrafish. Science (New York, NY) 298(5601):2188–2190. https://doi.org/10.1126/science.1077857
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN et al (2011) Transient regenerative potential of the neonatal mouse heart. Science (New York, NY) 331(6020):1078–1080. https://doi.org/10.1126/science.1200708
Williams C, Quinn KP, Georgakoudi I, Black LD 3rd. (2014) Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater 10(1):194–204. https://doi.org/10.1016/j.actbio.2013.08.037
Wang J, Karra R, Dickson AL, Poss KD (2013) Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev Biol 382(2):427–435. https://doi.org/10.1016/j.ydbio.2013.08.012
Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM et al (2009) Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell 16(2):233–244. https://doi.org/10.1016/j.devcel.2008.12.007
Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C et al (2017) Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation. 136(9):834–848. https://doi.org/10.1161/circulationaha.116.024307
Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S et al (2007) Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13(8):962–969. https://doi.org/10.1038/nm1619
Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD (2009) Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res 104(1):e1–e7. https://doi.org/10.1161/circresaha.108.188649
Chen Z, Xie J, Hao H, Lin H, Wang L, Zhang Y et al (2017) Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3beta/cyclin D1 signalling pathway. Cardiovasc Res 113(6):620–632. https://doi.org/10.1093/cvr/cvx001
Gupta V, Grande-Allen KJ (2006) Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res 72(3):375–383. https://doi.org/10.1016/j.cardiores.2006.08.017
Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E (2015) Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife. 4. https://doi.org/10.7554/eLife.07455
Akhyari P, Fedak PW, Weisel RD, Lee TY, Verma S, Mickle DA et al (2002) Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 106(12 Suppl 1):I137–I142
Birla RK, Huang YC, Dennis RG (2007) Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng 13(9):2239–2248. https://doi.org/10.1089/ten.2006.0359
Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S et al (2015) Human ventricular unloading induces cardiomyocyte proliferation. J Am Coll Cardiol 65(9):892–900. https://doi.org/10.1016/j.jacc.2014.12.027
Kong YP, Rioja AY, Xue X, Sun Y, Fu J, Putnam AJ (2018) A systems mechanobiology model to predict cardiac reprogramming outcomes on different biomaterials. Biomaterials. 181:280–292. https://doi.org/10.1016/j.biomaterials.2018.07.036
Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA et al (2020) An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 577(7790):405–409. https://doi.org/10.1038/s41586-019-1802-2
Bissell MJ, Aggeler J (1987) Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res 249:251–262
Pomeroy JE, Helfer A, Bursac N (2019) Biomaterializing the promise of cardiac tissue engineering. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2019.02.009
Wang F, Guan J (2010) Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Adv Drug Deliv Rev 62(7-8):784–797. https://doi.org/10.1016/j.addr.2010.03.001
Radisic M, Christman KL (2013) Materials science and tissue engineering: repairing the heart. Mayo Clin Proc 88(8):884–898. https://doi.org/10.1016/j.mayocp.2013.05.003
Christman KL, Lee RJ (2006) Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol 48(5):907–913. https://doi.org/10.1016/j.jacc.2006.06.005
Zimmermann WH, Melnychenko I, Eschenhagen T (2004) Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 25(9):1639–1647
Reis LA, Chiu LL, Feric N, Fu L, Radisic M (2016) Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med 10(1):11–28. https://doi.org/10.1002/term.1944
Johnson TD, Braden RL, Christman KL (2014) Injectable ECM scaffolds for cardiac repair. Methods Mol Biol (Clifton, NJ) 1181:109–120. https://doi.org/10.1007/978-1-4939-1047-2_10
Duan Y, Liu Z, O'Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G (2011) Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res 4(5):605–615. https://doi.org/10.1007/s12265-011-9304-0
Segers VF, Lee RT (2011) Biomaterials to enhance stem cell function in the heart. Circ Res 109(8):910–922. https://doi.org/10.1161/circresaha.111.249052
Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML et al (2017) Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 135(19):1832–1847. https://doi.org/10.1161/circulationaha.116.024145
Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C et al (2014) Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 74:151–161. https://doi.org/10.1016/j.yjmcc.2014.05.009
Nawroth JC, Scudder LL, Halvorson RT, Tresback J, Ferrier JP, Sheehy SP et al (2018) Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications. Biofabrication. 10(2):025004. https://doi.org/10.1088/1758-5090/aa96de
Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB et al (2013) Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 7(3):2369–2380. https://doi.org/10.1021/nn305559j
Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL et al (2015) Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 61:339–348. https://doi.org/10.1016/j.biomaterials.2015.05.005
Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI et al (2008) Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 14(2):213–221. https://doi.org/10.1038/nm1684
Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA (2014) Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS One 9(2):e90406. https://doi.org/10.1371/journal.pone.0090406
Tondreau MY, Laterreur V, Gauvin R, Vallieres K, Bourget JM, Lacroix D et al (2015) Mechanical properties of endothelialized fibroblast-derived vascular scaffolds stimulated in a bioreactor. Acta Biomater 18:176–185. https://doi.org/10.1016/j.actbio.2015.02.026
Wang Z, Long DW, Huang Y, Chen WCW, Kim K, Wang Y (2019) Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction. Acta Biomater 87:140–151. https://doi.org/10.1016/j.actbio.2019.01.062
Tang-Quan KR, Mehta NA, Sampaio LC, Taylor DA (2018) Whole cardiac tissue bioscaffolds. Adv Exp Med Biol 1098:85–114. https://doi.org/10.1007/978-3-319-97421-7_5
Daley MC, Fenn SL, Black LD 3rd. (2018) Applications of cardiac extracellular matrix in tissue engineering and regenerative medicine. Adv Exp Med Biol 1098:59–83. https://doi.org/10.1007/978-3-319-97421-7_4
Seo Y, Jung Y, Kim SH (2018) Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater 67:270–281. https://doi.org/10.1016/j.actbio.2017.11.046
Shudo Y, Cohen JE, MacArthur JW, Goldstone AB, Otsuru S, Trubelja A et al (2015) A tissue-engineered chondrocyte cell sheet induces extracellular matrix modification to enhance ventricular biomechanics and attenuate myocardial stiffness in ischemic cardiomyopathy. Tissue Eng A 21(19-20):2515–2525. https://doi.org/10.1089/ten.TEA.2014.0155
Lee KM, Kim H, Nemeno JG, Yang W, Yoon J, Lee S et al (2015) Natural cardiac extracellular matrix sheet as a biomaterial for cardiomyocyte transplantation. Transplant Proc 47(3):751–756. https://doi.org/10.1016/j.transproceed.2014.12.030
Ishii M, Shibata R, Shimizu Y, Yamamoto T, Kondo K, Inoue Y et al (2014) Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. Int J Cardiol 175(3):545–553. https://doi.org/10.1016/j.ijcard.2014.06.034
Yeh YC, Lee WY, Yu CL, Hwang SM, Chung MF, Hsu LW et al (2010) Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials. 31(25):6444–6453. https://doi.org/10.1016/j.biomaterials.2010.04.069
Wang CC, Chen CH, Lin WW, Hwang SM, Hsieh PC, Lai PH et al (2008) Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res 77(3):515–524. https://doi.org/10.1093/cvr/cvm046
Bracaglia LG, Winston S, Powell DA, Fisher JP (2019) Synthetic polymer coatings diminish chronic inflammation risk in large ECM-based materials. J Biomed Mater Res A 107(3):494–504. https://doi.org/10.1002/jbm.a.36564
Guan J, Wang F, Li Z, Chen J, Guo X, Liao J et al (2011) The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials. 32(24):5568–5580. https://doi.org/10.1016/j.biomaterials.2011.04.038
Loke WK, Khor E, Wee A, Teoh SH, Chian KS (1996) Hybrid biomaterials based on the interaction of polyurethane oligomers with porcine pericardium. Biomaterials. 17(22):2163–2172
Yang MC, Wang SS, Chou NK, Chi NH, Huang YY, Chang YL et al (2009) The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials. 30(22):3757–3765. https://doi.org/10.1016/j.biomaterials.2009.03.057
Zhang B, Xiao Y, Hsieh A, Thavandiran N, Radisic M (2011) Micro- and nanotechnology in cardiovascular tissue engineering. Nanotechnology. 22(49):494003. https://doi.org/10.1088/0957-4484/22/49/494003
Kim TG, Shin H, Lim DW (2012) Biomimetic scaffolds for tissue engineering. Adv Funct Mater 22(12):2446–2468. https://doi.org/10.1002/adfm.201103083
Boffito M, Di Meglio F, Mozetic P, Giannitelli SM, Carmagnola I, Castaldo C et al (2018) Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS One 13(7):e0199896. https://doi.org/10.1371/journal.pone.0199896
Kyburz KA, Anseth KS (2015) Synthetic mimics of the extracellular matrix: how simple is complex enough? Ann Biomed Eng 43(3):489–500. https://doi.org/10.1007/s10439-015-1297-4
Frydrych M, Roman S, MacNeil S, Chen B (2015) Biomimetic poly(glycerol sebacate)/poly(l-lactic acid) blend scaffolds for adipose tissue engineering. Acta Biomater 18:40–49. https://doi.org/10.1016/j.actbio.2015.03.004
Neal RA, McClugage SG, Link MC, Sefcik LS, Ogle RC, Botchwey EA (2009) Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng C Methods 15(1):11–21. https://doi.org/10.1089/ten.tec.2007.0366
Migliorini E, Thakar D, Sadir R, Pleiner T, Baleux F, Lortat-Jacob H et al (2014) Well-defined biomimetic surfaces to characterize glycosaminoglycan-mediated interactions on the molecular, supramolecular and cellular levels. Biomaterials. 35(32):8903–8915. https://doi.org/10.1016/j.biomaterials.2014.07.017
Hematti P (2018) Role of extracellular matrix in cardiac cellular therapies. Adv Exp Med Biol 1098:173–188. https://doi.org/10.1007/978-3-319-97421-7_9
Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL (2009) Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 30(29):5409–5416. https://doi.org/10.1016/j.biomaterials.2009.06.045
Wu WQ, Peng S, Song ZY, Lin S (2019) Collagen biomaterial for the treatment of myocardial infarction: an update on cardiac tissue engineering and myocardial regeneration. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-019-00627-0
Roura S, Galvez-Monton C, Bayes-Genis A (2017) Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J Tissue Eng Regen Med 11(8):2304–2313. https://doi.org/10.1002/term.2129
Rufaihah AJ, Seliktar D (2016) Hydrogels for therapeutic cardiovascular angiogenesis. Adv Drug Deliv Rev 96:31–39. https://doi.org/10.1016/j.addr.2015.07.003
Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ (2013) Decellularization for whole organ bioengineering. Biomed Mater (Bristol, England) 8(1):014106. https://doi.org/10.1088/1748-6041/8/1/014106
Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods (San Diego, Calif) 84:25–34. https://doi.org/10.1016/j.ymeth.2015.03.005
Taylor DA, Sampaio LC, Ferdous Z, Gobin AS, Taite LJ (2018) Decellularized matrices in regenerative medicine. Acta Biomater 74:74–89. https://doi.org/10.1016/j.actbio.2018.04.044
Spang MT, Christman KL (2018) Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater 68:1–14. https://doi.org/10.1016/j.actbio.2017.12.019
Badylak SF, Taylor D, Uygun K (2011) Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13:27–53. https://doi.org/10.1146/annurev-bioeng-071910-124743
Bejleri D, Davis ME (2019) Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthc Mater 8(5):e1801217. https://doi.org/10.1002/adhm.201801217
Schenke-Layland K, Rofail F, Heydarkhan S, Gluck JM, Ingle NP, Angelis E et al (2009) The use of three-dimensional nanostructures to instruct cells to produce extracellular matrix for regenerative medicine strategies. Biomaterials. 30(27):4665–4675. https://doi.org/10.1016/j.biomaterials.2009.05.033
Alrefai MT, Murali D, Paul A, Ridwan KM, Connell JM, Shum-Tim D (2015) Cardiac tissue engineering and regeneration using cell-based therapy. Stem Cells Cloning 8:81–101. https://doi.org/10.2147/sccaa.s54204
Sakaguchi K, Shimizu T, Okano T (2015) Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release 205:83–88. https://doi.org/10.1016/j.jconrel.2014.12.016
Cyranoski D (2018) ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature. 557(7707):619–620. https://doi.org/10.1038/d41586-018-05278-8
Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Funakoshi S et al (2017) Enhanced therapeutic effects of human iPS cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci Rep 7(1):8824. https://doi.org/10.1038/s41598-017-08869-z
Shimizu T, Sekine H, Yamato M, Okano T (2009) Cell sheet-based myocardial tissue engineering: new hope for damaged heart rescue. Curr Pharm Des 15(24):2807–2814
Matsuura K, Utoh R, Nagase K, Okano T (2014) Cell sheet approach for tissue engineering and regenerative medicine. J Control Release 190:228–239. https://doi.org/10.1016/j.jconrel.2014.05.024
Efraim Y, Sarig H, Cohen Anavy N, Sarig U, de Berardinis E, Chaw SY et al (2017) Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater 50:220–233. https://doi.org/10.1016/j.actbio.2016.12.015
Klouda L (2015) Thermoresponsive hydrogels in biomedical applications: a seven-year update. Eur J Pharm Biopharm 97(Pt B):338–349. https://doi.org/10.1016/j.ejpb.2015.05.017
Zhu J, Marchant RE (2011) Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Dev 8(5):607–626. https://doi.org/10.1586/erd.11.27
Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S (2012) Minimally invasive cell-seeded biomaterial systems for injectable/epicardial implantation in ischemic heart disease. Int J Nanomedicine 7:5969–5994. https://doi.org/10.2147/ijn.s37575
Lau HK, Kiick KL (2015) Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules. 16(1):28–42. https://doi.org/10.1021/bm501361c
Rufaihah AJ, Vaibavi SR, Plotkin M, Shen J, Nithya V, Wang J et al (2013) Enhanced infarct stabilization and neovascularization mediated by VEGF-loaded PEGylated fibrinogen hydrogel in a rodent myocardial infarction model. Biomaterials. 34(33):8195–8202. https://doi.org/10.1016/j.biomaterials.2013.07.031
Toba H, Lindsey ML (2019) Extracellular matrix roles in cardiorenal fibrosis: potential therapeutic targets for CVD and CKD in the elderly. Pharmacol Ther 193:99–120. https://doi.org/10.1016/j.pharmthera.2018.08.014
Yanamandala M, Zhu W, Garry DJ, Kamp TJ, Hare JM, Jun HW et al (2017) Overcoming the roadblocks to cardiac cell therapy using tissue engineering. J Am Coll Cardiol 70(6):766–775. https://doi.org/10.1016/j.jacc.2017.06.012
Zhu Y, Matsumura Y, Wagner WR (2017) Ventricular wall biomaterial injection therapy after myocardial infarction: advances in material design, mechanistic insight and early clinical experiences. Biomaterials. 129:37–53. https://doi.org/10.1016/j.biomaterials.2017.02.032
Chien KR, Frisen J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL (2019) Regenerating the field of cardiovascular cell therapy. Nat Biotechnol 37(3):232–237. https://doi.org/10.1038/s41587-019-0042-1
Kai D, Wang QL, Wang HJ, Prabhakaran MP, Zhang Y, Tan YZ et al (2014) Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater 10(6):2727–2738. https://doi.org/10.1016/j.actbio.2014.02.030
Wendel JS, Ye L, Zhang P, Tranquillo RT, Zhang JJ (2014) Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng A 20(7-8):1325–1335. https://doi.org/10.1089/ten.TEA.2013.0312
Gaballa MA, Sunkomat JN, Thai H, Morkin E, Ewy G, Goldman S (2006) Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J Heart Lung Transplant 25(8):946–954. https://doi.org/10.1016/j.healun.2006.04.008
Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A et al (2013) The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 34(36):9048–9055. https://doi.org/10.1016/j.biomaterials.2013.08.017
Deng C, Zhang P, Vulesevic B, Kuraitis D, Li F, Yang AF et al (2010) A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng A 16(10):3099–3109. https://doi.org/10.1089/ten.tea.2009.0504
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater (Deerfield Beach, Fla) 21(32-33):3307–3329. https://doi.org/10.1002/adma.200802106
Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA et al (2012) Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater (Deerfield Beach, Fla) 24(1):64–70, 2. https://doi.org/10.1002/adma.201103574
Saludas L, Pascual-Gil S, Prosper F, Garbayo E, Blanco-Prieto M (2017) Hydrogel based approaches for cardiac tissue engineering. Int J Pharm 523(2):454–475. https://doi.org/10.1016/j.ijpharm.2016.10.061
Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C et al (2014) 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater (Deerfield Beach, Fla) 26(1):85–123
Shi K, Wang YL, Qu Y, Liao JF, Chu BY, Zhang HP et al (2016) Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci Rep 6:19077. https://doi.org/10.1038/srep19077
Vo TN, Ekenseair AK, Spicer PP, Watson BM, Tzouanas SN, Roh TT et al (2015) In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering. J Control Release 205:25–34. https://doi.org/10.1016/j.jconrel.2014.11.028
Barnes AL, Genever PG, Rimmer S, Coles MC (2016) Collagen-poly(N-isopropylacrylamide) hydrogels with tunable properties. Biomacromolecules. 17(3):723–734. https://doi.org/10.1021/acs.biomac.5b01251
Das D, Ghosh P, Ghosh A, Haldar C, Dhara S, Panda AB et al (2015) Stimulus-responsive, biodegradable, biocompatible, covalently cross-linked hydrogel based on dextrin and poly(N-isopropylacrylamide) for in vitro/in vivo controlled drug release. ACS Appl Mater Interfaces 7(26):14338–14351. https://doi.org/10.1021/acsami.5b02975
Chen FM, Liu X (2016) Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 53:86–168. https://doi.org/10.1016/j.progpolymsci.2015.02.004
Hastings CL, Roche ET, Ruiz-Hernandez E, Schenke-Layland K, Walsh CJ, Duffy GP (2015) Drug and cell delivery for cardiac regeneration. Adv Drug Deliv Rev 84:85–106. https://doi.org/10.1016/j.addr.2014.08.006
Wang H, Zhou J, Liu Z, Wang C (2010) Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med 14(5):1044–1055. https://doi.org/10.1111/j.1582-4934.2010.01046.x
Ungerleider JL, Johnson TD, Rao N, Christman KL (2015) Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods (San Diego, Calif) 84:53–59. https://doi.org/10.1016/j.ymeth.2015.03.024
Shu Y, Hao T, Yao F, Qian Y, Wang Y, Yang B et al (2015) RoY peptide-modified chitosan-based hydrogel to improve angiogenesis and cardiac repair under hypoxia. ACS Appl Mater Interfaces 7(12):6505–6517. https://doi.org/10.1021/acsami.5b01234
Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM et al (2012) Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol 59(8):751–763. https://doi.org/10.1016/j.jacc.2011.10.888
Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A et al (2012) Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today 42(2):181–184. https://doi.org/10.1007/s00595-011-0106-4
Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E et al (2004) Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351(12):1187–1196. https://doi.org/10.1056/NEJMoa040455
Sato M, Yamato M, Hamahashi K, Okano T, Mochida J (2014) Articular cartilage regeneration using cell sheet technology. Anat Rec (Hoboken, NJ : 2007) 297(1):36–43. https://doi.org/10.1002/ar.22829
Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H et al (2006) Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 55(12):1704–1710. https://doi.org/10.1136/gut.2005.088518
Itabashi Y, Miyoshi S, Yuasa S, Fujita J, Shimizu T, Okano T et al (2005) Analysis of the electrophysiological properties and arrhythmias in directly contacted skeletal and cardiac muscle cell sheets. Cardiovasc Res 67(3):561–570. https://doi.org/10.1016/j.cardiores.2005.03.014
Itabashi Y, Miyoshi S, Kawaguchi H, Yuasa S, Tanimoto K, Furuta A et al (2005) A new method for manufacturing cardiac cell sheets using fibrin-coated dishes and its electrophysiological studies by optical mapping. Artif Organs 29(2):95–103. https://doi.org/10.1111/j.1525-1594.2005.29020.x
Matsuura K, Wada M, Shimizu T, Haraguchi Y, Sato F, Sugiyama K et al (2012) Creation of human cardiac cell sheets using pluripotent stem cells. Biochem Biophys Res Commun 425(2):321–327. https://doi.org/10.1016/j.bbrc.2012.07.089
Lee P, Klos M, Bollensdorff C, Hou L, Ewart P, Kamp TJ et al (2012) Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res 110(12):1556–1563. https://doi.org/10.1161/circresaha.111.262535
Fujita J, Itabashi Y, Seki T, Tohyama S, Tamura Y, Sano M et al (2012) Myocardial cell sheet therapy and cardiac function. Am J Physiol Heart Circ Physiol 303(10):H1169–H1182. https://doi.org/10.1152/ajpheart.00376.2012
Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C et al (2012) Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation. 125(1):140–149. https://doi.org/10.1161/circulationaha.111.049072
Kofidis T, de Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T et al (2004) Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg 128(4):571–578. https://doi.org/10.1016/j.jtcvs.2004.05.021
Iyisoy A, Ozturk C, Celik T, Demirkol S, Cingoz F, Unlu M et al (2015) Percutaneous transapical closure of cardiac apex with an ADO-II device after successful transapical transcatheter prosthetic mitral paravalvular leak closure. Int J Cardiol 189:289–292. https://doi.org/10.1016/j.ijcard.2015.04.076
Wu KH, Liu YL, Zhou B, Han ZC (2006) Cellular therapy and myocardial tissue engineering: the role of adult stem and progenitor cells. Eur J Cardiothorac Surg 30(5):770–781. https://doi.org/10.1016/j.ejcts.2006.08.003
Aamodt JM, Grainger DW (2016) Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 86:68–82. https://doi.org/10.1016/j.biomaterials.2016.02.003
Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha BH, Vishwakarma A, Ghaemmaghami AM et al (2016) Delivery strategies to control inflammatory response: modulating M1-M2 polarization in tissue engineering applications. J Control Release 240:349–363. https://doi.org/10.1016/j.jconrel.2016.01.026
Du C, Cui FZ, Zhu XD, de Groot K (1999) Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J Biomed Mater Res 44(4):407–415
Badylak SF (2007) The extracellular matrix as a biologic scaffold material. Biomaterials. 28(25):3587–3593. https://doi.org/10.1016/j.biomaterials.2007.04.043
Bouten CV, Dankers PY, Driessen-Mol A, Pedron S, Brizard AM, Baaijens FP (2011) Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev 63(4-5):221–241. https://doi.org/10.1016/j.addr.2011.01.007
Bracaglia LG, Fisher JP (2015) Extracellular matrix-based biohybrid materials for engineering compliant, matrix-dense tissues. Adv Healthc Mater 4(16):2475–2487. https://doi.org/10.1002/adhm.201500236
Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE (2016) In vitro cardiac tissue models: current status and future prospects. Adv Drug Deliv Rev 96:203–213. https://doi.org/10.1016/j.addr.2015.09.011
Leor J, Amsalem Y, Cohen S (2005) Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther 105(2):151–163. https://doi.org/10.1016/j.pharmthera.2004.10.003
Berglund JD, Galis ZS (2003) Designer blood vessels and therapeutic revascularization. Br J Pharmacol 140(4):627–636. https://doi.org/10.1038/sj.bjp.0705457
Tedder ME, Liao J, Weed B, Stabler C, Zhang H, Simionescu A et al (2009) Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng A 15(6):1257–1268. https://doi.org/10.1089/ten.tea.2008.0263
Arts T, Bovendeerd PH, Prinzen FW, Reneman RS (1991) Relation between left ventricular cavity pressure and volume and systolic fiber stress and strain in the wall. Biophys J 59(1):93–102. https://doi.org/10.1016/s0006-3495(91)82201-9
Mol A, van Lieshout MI, Dam-de Veen CG, Neuenschwander S, Hoerstrup SP, Baaijens FP et al (2005) Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials. 26(16):3113–3121. https://doi.org/10.1016/j.biomaterials.2004.08.007
Langer R, Vacanti JP (1993) Tissue engineering. Science. 260(5110):920–926
Fuchs JR, Nasseri BA, Vacanti JP (2001) Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg 72(2):577–591
Ishii O, Shin M, Sueda T, Vacanti JP (2005) In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J Thorac Cardiovasc Surg 130(5):1358–1363. https://doi.org/10.1016/j.jtcvs.2005.05.048
Freytes DO, Wan LQ, Vunjak-Novakovic G (2009) Geometry and force control of cell function. J Cell Biochem 108(5):1047–1058. https://doi.org/10.1002/jcb.22355
Adams JC, Watt FM (1993) Regulation of development and differentiation by the extracellular matrix. Development (Cambridge, England) 117(4):1183–1198
Brown RE, Butler JP, Rogers RA, Leith DE (1994) Mechanical connections between elastin and collagen. Connect Tissue Res 30(4):295–308
Duffy GP, McFadden TM, Byrne EM, Gill SL, Farrell E, O'Brien FJ (2011) Towards in vitro vascularisation of collagen-GAG scaffolds. Eur Cells Mater 21:15–30
Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R et al (2004) Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A 101(52):18129–18134. https://doi.org/10.1073/pnas.0407817101
Wei HJ, Chen CH, Lee WY, Chiu I, Hwang SM, Lin WW et al (2008) Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 29(26):3547–3556. https://doi.org/10.1016/j.biomaterials.2008.05.009
Huebsch N, Mooney DJ (2009) Inspiration and application in the evolution of biomaterials. Nature. 462(7272):426–432. https://doi.org/10.1038/nature08601
Williams DF (2009) On the nature of biomaterials. Biomaterials. 30(30):5897–5909. https://doi.org/10.1016/j.biomaterials.2009.07.027
Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW et al (2010) Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng C Methods 16(3):525–532. https://doi.org/10.1089/ten.TEC.2009.0392
Ismagilov RF, Maharbiz MM (2007) Can we build synthetic, multicellular systems by controlling developmental signaling in space and time? Curr Opin Chem Biol 11(6):604–611. https://doi.org/10.1016/j.cbpa.2007.10.003
Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U et al (2009) Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol 54(11):1014–1023. https://doi.org/10.1016/j.jacc.2009.06.010
Martens TP, Godier AF, Parks JJ, Wan LQ, Koeckert MS, Eng GM et al (2009) Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transplant 18(3):297–304. https://doi.org/10.3727/096368909788534915
Rodell CB, Lee ME, Wang H, Takebayashi S, Takayama T, Kawamura T et al (2016) Injectable shear-thinning hydrogels for minimally invasive delivery to infarcted myocardium to limit left ventricular remodeling. Circ Cardiovasc Interv 9(10). https://doi.org/10.1161/circinterventions.116.004058
Johnson TD, Christman KL (2013) Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv 10(1):59–72. https://doi.org/10.1517/17425247.2013.739156
Dib N, Campbell A, Jacoby DB, Zawadzka A, Ratliff J, Miedzybrocki BM et al (2006) Safety and feasibility of percutaneous autologous skeletal myoblast transplantation in the coil-infarcted swine myocardium. J Pharmacol Toxicol Methods 54(1):71–77. https://doi.org/10.1016/j.vascn.2005.12.002
Rao SV, Zeymer U, Douglas PS, Al-Khalidi H, Liu J, Gibson CM et al (2015) A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: Rationale and design of the PRESERVATION I trial. Am Heart J 170(5):929–937. https://doi.org/10.1016/j.ahj.2015.08.017
Rao SV, Zeymer U, Douglas PS, Al-Khalidi H, White JA, Liu J et al (2016) Bioabsorbable intracoronary matrix for prevention of ventricular remodeling after myocardial infarction. J Am Coll Cardiol 68(7):715–723. https://doi.org/10.1016/j.jacc.2016.05.053
Mann DL, Lee RJ, Coats AJ, Neagoe G, Dragomir D, Pusineri E et al (2016) One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail 18(3):314–325. https://doi.org/10.1002/ejhf.449
Mewhort HE, Turnbull JD, Satriano A, Chow K, Flewitt JA, Andrei AC et al (2016) Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. J Heart Lung Transplant 35(5):661–670. https://doi.org/10.1016/j.healun.2016.01.012
Mewhort HE, Turnbull JD, Meijndert HC, Ngu JM, Fedak PW (2014) Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J Thorac Cardiovasc Surg 147(5):1650–1659. https://doi.org/10.1016/j.jtcvs.2013.08.005
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A et al (2018) Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 71(4):429–438. https://doi.org/10.1016/j.jacc.2017.11.047
Sahoo S, Losordo DW (2014) Exosomes and cardiac repair after myocardial infarction. Circ Res 114(2):333–344. https://doi.org/10.1161/circresaha.114.300639
Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J et al (2018) Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng 2(5):293–303. https://doi.org/10.1038/s41551-018-0229-7
Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN et al (2014) Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater 13(6):653–661. https://doi.org/10.1038/nmat3922
Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M et al (2017) Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng 1:983–992. https://doi.org/10.1038/s41551-017-0157-y
Fan Z, Xu Z, Niu H, Gao N, Guan Y, Li C et al (2018) An injectable oxygen release system to augment cell survival and promote cardiac repair following myocardial infarction. Sci Rep 8(1):1371. https://doi.org/10.1038/s41598-018-19906-w
Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA et al (2017) Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun 8:13724. https://doi.org/10.1038/ncomms13724
Funding
This research was funded by Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-015); National Natural Science Foundation of China (NSFC, 81770308, 81500239); the National Key Research and Development Project of China (2019YFA0801500); and CAMS Beijing Natural Science Foundation (7172183).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.N.; Writing: H.L., M.B., and Y.N.; Editing: H.L. and Y.N.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Bao, M. & Nie, Y. Extracellular matrix–based biomaterials for cardiac regeneration and repair. Heart Fail Rev 26, 1231–1248 (2021). https://doi.org/10.1007/s10741-020-09953-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09953-9