Abstract
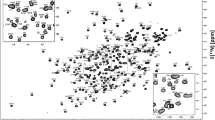
TGIF1 is an essential regulator of cell differentiation in various biological processes, and is associated with holoprosencephaly and many cancers. The C-terminal domain of TGIF1 that was originally defined as repressive domain 2 can interact with a variety of proteins, such as transcription factor Smad2 and co-repressor Sin3A, to mediate the regulative roles of TGIF1 in diverse cell signaling pathways. However, the recognition mechanism of TGIF1 C-terminal domain for different interacting proteins remains unknown. Here, we report the nearly complete 1H, 13C, and 15N backbone and side chain resonance assignments of TGIF1 C-terminal domain (residues 256–375), laying a foundation for further research on the structure–function relationship of TGIF1.


Similar content being viewed by others
References
Bertolino E, Reimund B, Wildt-Perinic D, Clerc RG (1995) A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J Biol Chem 270:31178–31188
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Demange C, Ferrand N, Prunier C, Bourgeade MF, Atfi A (2009) A model of partnership co-opted by the homeodomain protein TGIF and the Itch/AIP4 ubiquitin ligase for effective execution of TNF-alpha cytotoxicity. Mol Cell 36:1073–1085
El-Jaick KB, Powers SE, Bartholin L, Myers KR, Hahn J, Orioli IM, Ouspenskaia M, Lacbawan F, Roessler E, Wotton D, Muenke M (2007) Functional analysis of mutations in TGIF associated with holoprosencephaly. Mol Genet Metab 90:97–111
Ettahar A, Ferrigno O, Zhang MZ, Ohnishi M, Ferrand N, Prunier C, Levy L, Bourgeade MF, Bieche I, Romero DG, Colland F, Atfi A (2013) Identification of PHRF1 as a tumor suppressor that promotes the TGF-beta cytostatic program through selective release of TGIF-driven PML inactivation. Cell Rep 4:530–541
Ferrand N, Demange C, Prunier C, Seo SR, Atfi A (2007) A mechanism for mutational inactivation of the homeodomain protein TGIF in holoprosencephaly. FASEB J 21:488–496
Guca E, Sunol D, Ruiz L, Konkol A, Cordero J, Torner C, Aragon E, Martin-Malpartida P, Riera A, Macias MJ (2018) TGIF1 homeodomain interacts with Smad MH1 domain and represses TGF-beta signaling. Nucleic Acids Res 46:9220–9235
Hamid R, Brandt SJ (2009) Transforming growth-interacting factor (TGIF) regulates proliferation and differentiation of human myeloid leukemia cells. Mol Oncol 3:451–463
Li S, Hu R, Yao H, Long D, Luo F, Zhou X, Zhang X, Liu M, Zhu J, Yang Y (2018) Characterization of the interaction interface and conformational dynamics of human TGIF1 homeodomain upon the binding of consensus DNA. Biochim Biophys Acta Proteins Proteom 1866:1021–1028
Lo RS, Wotton D, Massague J (2001) Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J 20:128–136
Melhuish TA, Wotton D (2000) The interaction of the carboxyl terminus-binding protein with the Smad corepressor TGIF is disrupted by a holoprosencephaly mutation in TGIF. J Biol Chem 275:39762–39766
Saito H, Gasser A, Bolamperti S, Maeda M, Matthies L, Jahn K, Long CL, Schluter H, Kwiatkowski M, Saini V, Pajevic PD, Bellido T, van Wijnen AJ, Mohammad KS, Guise TA, Taipaleenmaki H, Hesse E (2019) TG-interacting factor 1 (Tgif1)-deficiency attenuates bone remodeling and blunts the anabolic response to parathyroid hormone. Nat Commun 10:1354
Seo SR, Lallemand F, Ferrand N, Pessah M, L’Hoste S, Camonis J, Atfi A (2004) The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J 23:3780–3792
Shah A, Melhuish TA, Fox TE, Frierson HF Jr, Wotton D (2019) TGIF transcription factors repress acetyl CoA metabolic gene expression and promote intestinal tumor growth. Genes Dev 33:388–402
Sharma M, Sun Z (2001) 5′TG3′ interacting factor interacts with Sin3A and represses AR-mediated transcription. Mol Endocrinol 15:1918–1928
Shen Y, Bax A (2013) Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 56:227–241
Taniguchi K, Anderson AE, Sutherland AE, Wotton D (2012) Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway. PLoS Genet 8:e1002524
Wotton D, Lo RS, Lee S, Massague J (1999) A Smad transcriptional corepressor. Cell 97:29–39
Yeh BW, Wu WJ, Li WM, Li CC, Huang CN, Kang WY, Liu ZM, Huang HS (2012) Overexpression of TG-interacting factor is associated with worse prognosis in upper urinary tract urothelial carcinoma. Am J Pathol 181:1044–1055
Zhang MZ, Ferrigno O, Wang Z, Ohnishi M, Prunier C, Levy L, Razzaque M, Horne WC, Romero D, Tzivion G, Colland F, Baron R, Atfi A (2015) TGIF governs a feed-forward network that empowers Wnt signaling to drive mammary tumorigenesis. Cancer Cell 27:547–560
Zhu J, Li S, Ramelot TA, Kennedy MA, Liu M, Yang Y (2018) Structural insights into the impact of two holoprosencephaly-related mutations on human TGIF1 homeodomain. Biochem Biophys Res Commun 496:575–581
Acknowledgements
We thank for grant supports from the National Natural Science Foundation of China (Grant Numbers: 21575155 and 21703283).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, C., Nie, Y., Yue, X. et al. Backbone and side chain resonance assignments of the C-terminal domain of human TGIF1. Biomol NMR Assign 13, 357–360 (2019). https://doi.org/10.1007/s12104-019-09905-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-019-09905-x