Abstract
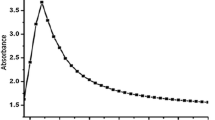
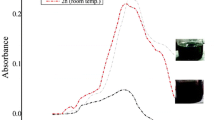
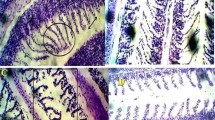
Silver nanoparticles (Ag-NPs) have wide applications in the medical field; however, the toxicological effects are still poorly studied. The study was aimed to determine the effects of 15.78 nm spherical and amine-coated Ag-NPs on hematology and histology of gills and liver tissues in 28 days treated Labeo rohita (L. rohita). It was found that Ag-NPs induced alterations in the hematological parameters in a dose dependent manner. The Ag-NPs also induced histological alterations in a dose-dependent manner. In gill tissues, it induced fusion of secondary lamellae, separation of gill epithelium, fusion and necrosis of lamellar cells, hyperplasia, deformed cartilaginous skeleton, separation and lifting of epithelium, and curling of lamellae in a dose dependent manner. In the liver, Ag-NPs produced abnormalities in hepatic tissues by reducing the size of hepatocytes and nuclei, and stimulated the production of necrotic and apoptotic bodies. It was concluded that Ag-NPs are toxic to aquatic organisms and induce hematotoxicity and histopathological conditions in exposed fish.








Similar content being viewed by others
Change history
27 February 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12011-024-04124-5
References
Völker C, Oetken M, Oehlmann J (2013) The biological effects and possible modes of action of nanosilver, in reviews of environmental contamination and toxicology volume 223, Springer. p. 81–106. doi: 10.1007/978-1-4614-5577-6_4
Woodrow Wilson dWoodrow Wilson d (2016) Nanotechnology consumer product inventory [cited 2016 28 Aprthe case; Available from: http://www.nanotechproject.org/cpi/about/analysis.
Khan MS, Jabeen F, Qureshi NA, Asghar MS, Shakeel M, Noureen A (2015a) Toxicity of silver nanoparticles in fish: a critical review. J Bio Environ Sci 6(5):211–227
Schluesener JK, Schluesener HJ (2013) Nanosilver: application and novel aspects of toxicology. Arch Toxicol 87(4):569–576. doi:10.1007/s00204-012-1007-z
Taju G, Majeed SA, Nambi K, Hameed AS (2014) In vitro assay for the toxicity of silver nanoparticles using heart and gill cell lines of Catla catla and gill cell line of Labeo rohita. Comp Biochem Physiol C Pharmacol Toxicol 161:41–52. doi:10.1016/j.cbpc.2014.01.007
Awasthi KK, Awasthi A, Bhoot N, John P, Sharma SK, Awasthi K (2013) Antimicrobial properties of electro-chemically stabilized organo-metallic thin films. Adv Electrochem 1(1):42–47. doi:10.1166/adel.2013.1013
Smith IC, Carson BL (1977) Trace metals in the environment. Vol. 1. Arbor Science Publishers, USA
Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG, Heugens EH, Roszek B, Bisschops J, Gosens I, Van De Meent D (2009) Nano-silver—a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicol 3(2):109–138. doi:10.1080/17435390902725914
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45(6):2360–2367. doi:10.1021/es103995x
Khan MS, Jabeen F, Asghar MS, Qureshi NA, Shakeel M, Noureen A, Shabbir S (2015) Role of nao-ceria in the amelioration of oxidative stress: current and future applications in medicine. Int J Biosci 6(8):89–109. doi:10.12692/ijb/6.8.89-109
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22
Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112(43):13608–13619
K-i I, Takano H, Yanagisawa R, Koike E, Shimada A (2009) Size effects of latex nanomaterials on lung inflammation in mice. Toxicol Appl Pharmacol 234(1):68–76
Parish C (2013) Agency for toxic substances and disease registry doi: 10.1.1.361.6740
Wan AT, Conyers R, Coombs CJ, Masterton JP (1991) Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin Chem 37(10):1683–1687
Larese FF, D’Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, Maina G (2009) Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 255(1):33–37. doi:10.1016/j.tox.2008.09.025
Khan MS, Qureshi NA, Jabeen F, Asghar MS, Shakeel M, Fakhar-E-Alam M (2016) Eco-friendly synthesis of silver nanoparticles through economical methods and assessment of toxicity through oxidative stress analysis in the Labeo Rohita. Biol Trace Elem Res:1–13. doi:10.1007/s12011-016-0838-5
Ali D (2014) Oxidative stress-mediated apoptosis and genotoxicity induced by silver nanoparticles in freshwater snail Lymnea luteola L. Biol Trace Elem Res 162(1–3):333–341. doi:10.1007/s12011-014-0158-6
Arora S, Jain J, Rajwade J, Paknikar K (2009) Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol 236(3):310–318. doi:10.1016/j.taap.2009.02.020
Zhornik E, Baranova L, Drozd E, Sudas M, Chau N, Buu N, Dung T, Chizhik S, Volotovski I (2014) Silver nanoparticles induce lipid peroxidation and morphological changes in human lymphocytes surface. Biophys 59(3):380–386. doi:10.1134/s0006350914030282
Schrand AM, Braydich-Stolle LK, Schlager JJ, Dai L, Hussain SM (2008) Can silver nanoparticles be useful as potential biological labels? Nanotech 19(23):235104. doi:10.1088/0957-4484/19/23/235104
Ahamed M, Alsalhi MS, Siddiqui MK (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411(23–24):1841–1848. doi:10.1016/j.cca.2010.08.016
Zhang T, Wang L, Chen Q, Chen C (2014) Cytotoxic potential of silver nanoparticles. Yonsei Med J 55(2):283–291. doi:10.3349/ymj.2014.55.2.283
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett 201(1):92–100. doi:10.1016/j.toxlet.2010.12.010
Hussain S, Hess K, Gearhart J, Geiss K, Schlager J (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro 19(7):975–983. doi:10.1016/j.tiv.2005.06.034
Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Kim J (2008) Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal Toxicol 20(6):567–574. doi:10.1080/08958370701874671
Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, Mattiello S, Cubadda F, Aureli F, D’Amato M, Raggi A (2016) Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol 13(1):1
Kataria N, Kataria AK, Pandey N, Gupta P (2010) Serum biomarkers of physiological defense against reactive oxygen species during environmental stress in Indian dromedaries. HVM Bioflux 2(2):55–60
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci 24(6):1091–1098. doi:10.1016/S1001-0742(11)60845-0
Lee B, Duong CN, Cho J, Lee J, Kim K, Seo Y, Kim P, Choi K, Yoon J (2012, 2012) Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). Biomed Res Int
Afifi M, Saddick S, Zinada OAA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23(6):754–760. doi:10.1016/j.sjbs.2016.06.008
Li S-D, Huang L (2008) Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 5(4):496–504. doi:10.1021/mp800049w
Dobšíková R, Svobodová Z, Blahova J, Modrá H, Velíšek J (2006) Stress response to long distance transportation of common carp (Cyprinus carpio L.) Acta Vet Brno 75(3):437–448
Dobšíková R, Svobodova Z, Blahova J, Modra H, Velíšek J (2009) The effect of transport on biochemical and haematological indices of common carp (Cyprinus carpio L.) Czech J Anim Sci 54(11):510–518
Di Giulio RT, Hinton DE (2008) The toxicology of fishes. Crc Press. doi:10.1201/9780203647295
Imani M, Halimi M, Khara H (2015) Effects of silver nanoparticles (AgNPs) on hematological parameters of rainbow trout, Oncorhynchus mykiss. Comp Clin Pathol 24(3):491–495. doi:10.1007/s00580-014-1927-5
Ruane N, Bonga SW, Balm P (1999) Differences between rainbow trout and brown trout in the regulation of the pituitary–interrenal axis and physiological performance during confinement. Gen Comp Endocrinol 115(2):210–219. doi:10.1006/gcen.1999.7292
Witeska M, Kościuk B (2003) The changes in common carp blood after short-term zinc exposure. Environ Sci Pollut R 10(5):284–286. doi:10.1065/espr2003.07.161
Vutukuru S (2005) Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeo rohita. Int J Environ Res Publ Health 2(3):456–462. doi:10.3390/ijerph2005030010
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (common carp). Int J Environ Sci Tech 5(2):179–182. doi:10.1007/bf03326011
Stoskopf KM (1993) Fish medicine, 1st edition. W.B. Saunders Co., Philadelphia
Vandebriel RJ, Tonk EC, de la Fonteyne-Blankestijn LJ, Gremmer ER, Verharen HW, van der Ven LT, van Loveren H, de Jong WH (2014) Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part Fibre Toxicol 11(1):1–9. doi:10.1186/1743-8977-11-21
Adams SM (2002) Biological indicators of aquatic ecosystem stress. American Fisheries Society.
Cheraghi J, Hosseini E, Hoshmandfar R, Sahraei R (2013) Hematologic parameters study of male and female rats administrated with different concentrations of silver nanoparticles. Intl J Agri Crop Sci 5(7):789
Ellsaesser C, Clem L (1986) Haematological and immunological changes in channel catfish stressed by handling and transport. J Fish Biol 28(4):511–521. doi:10.1016/0145-305x(86)90149-7
Ikramullah A, Salve D, Pai G, Rathore M, Joshi D (2013) In vitro cytotoxicity testing of silver nano-particals in lymphocyte and sperm cells. Ind J Fund Appl Life Sci 3:44–47
Banaee M, Mirvagefei A, Rafei G, Majazi Amiri B (2008) Effect of sub-lethal diazinon concentrations on blood plasma biochemistry. Int J Environ Res 12(2):189–198
Abarghoei S, Hedayati SA, Ghafari Farsani H, Gerami MH (2015) Hematological responses of goldfish (Carassius auratus) to different acute concentrations of silver sulfate as a toxicant. Pollution 1(3):247–256. doi:10.7508/pj.2015.03.001
Williams KM, Gokulan K, Cerniglia CE, Khare S (2016) Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J Nanobiotechnology 14(1):62. doi:10.1186/s12951-016-0214-9
Soares T, Ribeiro D, Proença C, Chisté RC, Fernandes E, Freitas M (2016) Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci 145:247–254. doi:10.1016/j.lfs.2015.12.046
Liz R, Simard J-C, Leonardi LBA, Girard D (2015) Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int Immunopharmacol 28(1):616–625. doi:10.1016/j.intimp.2015.06.030
Neumann NF, Barreda DR, Belosevic M (2000) Generation and functional analysis of distinct macrophage sub-populations from goldfish (Carassius auratus L.) kidney leukocyte cultures. Fish Shellfish Immunol 10(1):1–20. doi:10.1006/fsim.1999.0221
Rieger AM, Hall BE, Barreda DR (2010) Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev Comp Immunol 34(11):1144–1159. doi:10.1016/j.dci.2010.06.006
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115. doi:10.1016/j.aquatox.2012.10.005
Rajkumar K, Kanipandian N, Thirumurugan R (2015) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci: 1–11. doi: 10.1007/s13204-015-0417-7
Al-Ghanbousi R, Ba-Omar T, Victor R (2012) Effect of deltamethrin on the gills of Aphanius dispar: a microscopic study. Tissue Cell 44(1):7–14. doi:10.1016/j.tice.2011.09.003
Van Dyk J, Marchand M, Pieterse G, Barnhoorn IE, Bornman M (2009) Histological changes in the gills of Clarias gariepinus (Teleostei: Clariidae) from a polluted south African urban aquatic system. Afr J Aquat Sci 34(3):283–291. doi:10.2989/ajas.2009.34.3.10.986
Perera S, Pathiratne A (2012) Haemato-immunological and histological responses in Nile tilapia, Oreochromis niloticus exposed to titanium dioxide nanoparticles. Sri Lanka J Aquat Sc 17: 1–18. Doi: 0.4038/sljas.v17i0.6852
Salah M, Farghali AA, Azmy H, Khedr MH (2013) Biological compatibility of carbon nanotubes for treatment of pollution of Nile tilapia (Oreochromis niloticus) by lead acetate. Life Sci J 10(2)
Wu Y, Zhou Q (2013) Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem 32(1):165–173. doi:10.1002/etc.2038
Sharifian M, Khani F, Khosravi K, Khalili M, Hedayati A (2013) Sublethal effect of nanosilver on the structure of gill of Caspian roach (Rutilus rutilus caspicus) fingerlings. Intl J Aquat Biol 1(2):55–60
Patel J, Bahadur A (2011) Histopathological manifestations of sub lethal toxicity of copper ions in Catla catla. Am-Eurasian J Toxicol Sci 4(1):01–05
Monfared AL, Soltani S (2013) Effects of silver nanoparticles administration on the liver of rainbow trout (Oncorhynchus mykiss): histological and biochemical studies. Eur J Exp Biol 3(2):285–289
Lee O, Green JM, Tyler CR (2015) Transgenic fish systems and their application in ecotoxicology. Crit Rev Toxicol 45(2):124–141. doi:10.3109/10408444.2014.965805
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s12011-024-04124-5
About this article
Cite this article
Khan, M.S., Qureshi, N.A., Jabeen, F. et al. RETRACTED ARTICLE: Assessment of Waterborne Amine-Coated Silver Nanoparticle (Ag-NP)-Induced Toxicity in Labeo rohita by Histological and Hematological Profiles. Biol Trace Elem Res 182, 130–139 (2018). https://doi.org/10.1007/s12011-017-1080-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1080-5