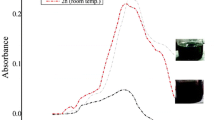
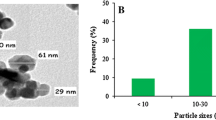
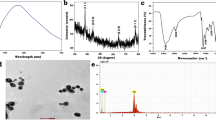
Abstract
Silver is one of the most toxic metals to freshwater aquatic organisms. Limited efforts have been made to study apoptosis and genotoxic potential of silver nanoparticles (AgNPs) in freshwater snail Lymnea luteola L. (L. luteola). Therefore, the present investigation was aimed to study the induction of apoptosis and DNA damage by AgNPs in L. luteola. AgNPs showed molluscicidal activity against L. luteola and three concentrations of AgNPs were selected, the concentration I (4 μg/l), concentration II (12 μg/l), and the concentration III (24 μg/l). Induction of oxidative stress in snail hemolymph was observed by a decrease in reduced glutathione (GSH) and glutathione S-transferase (GST) levels at different concentration of AgNPs, and on the other hand, malondialdehyde (MDA) levels increased at lower concentrations but decreased in higher concentration of AgNPs. Catalase (CAT) activity was also decreased at lower concentrations and increased in higher concentration of AgNPs. Flow cytometry data showed that AgNPs exposed hemocyte cells promote apoptotic and necrotic-mediated cell death when AgNPs concentrations were 12 and 24 μg/l compared to control. DNA damage scores increased with the exposure levels of AgNPs, and dose- and time-dependent effects were observed. A significant positive correlation was observed among reactive oxygen species (ROS) generation, apoptosis, and DNA damage. The study suggests that ROS may be involved in inducing apoptosis and DNA damage in the AgNPs exposed hemocyte cells of L. luteola. This study demonstrates that AgNPs is lethal to freshwater snail L. luteola.





Similar content being viewed by others
References
Oberdorster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Sci Technol 32:967–976
Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert M, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB (2006) Safe handling of nanotechnology. Nature 444:267–269
Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139
Mersch J, Beauvais MN, Nagel P (1996) Induction of micronuclei in haemocytes and gill cells of zebra mussels, Dreissena polymorpha, exposed to clastogens. Mutat Res 371:47–55
Lin J, Zhang H, Chen Z, Zheng Y (2010) Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 4(9):5421–5429
Mueller NC, Nowack B (2008) Exposure modelling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Purcell TW, Peters JJ (1998) Sources of silver in the environment. Environ Toxicol Chem 17:539–546
Rozan TF, Hunter KS, Benoit G (1995) Silver in fresh water: sources, transport and fate in Connecticut rivers. In: Proceedings of the 3th argentum international conference on the transport, fate and effects of silver in the environment, Washington, DC, USA, August 6–9:181–184
Wen LS, Santschi PH, Gill GA, Paternostro CL, Lehman RD (1997) Colloidal and particulate silver in river and estuarine waters of Texas. Environ Sci Techno 31:723–731
Simon KS, Simon MA, Benfield EF (2009) Variation in ecosystem function in Appalachian streams along an acidity gradient. Ecol Appl 19:1147–1160
Regoli F, Gorbi S, Frenzilli G, Nigro M, Cors I, Forcardi S, Winston GW (2002) Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Mar Environ Res 54:419–423
Kovochich M, Xia T, Xu J, Yeh JI, Nel AE (2007) Principles and procedures to assess nanomaterial toxicity. In: Wiesner MR, Bottero JY (eds) Environmental nanotechnology. Applications and impacts of nanomaterials. McGraw Hill, New York, pp 205–229
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmenn A, Kobatashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Muta 35:206–221
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B (2008) Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere 71:1823–1831
APHA, AWWA, WPCF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Publication of Health Association, Washington, DC
Finney DJ (1971) Probit analysis 3rd ed. Cambridge University Press, London, p 318
Nair V, Turner GE (1984) The thiobarbituric acid test for lipid peroxidation structure of the adduct with malondialdehyde. Lipids 19:84–95
Owens WI, Belcher RV (1965) A colorimetric micro-method for the determination of glutathione. Bioch J 94:705–711
Beers RF Jr, Sizers IW (1952) Spectrophotometric method for measuring the break-down of hydrogen peroxide by catalase. J of Bio Chem 195:133–140
Vessey DA, Boyer TD (1984) Differential activation and inhibition of different forms of rat liver glutathione S-transferase by the herbicides 2,4-dichloro phenoxy acetate (2,4-D) and 2,4, strichloro phenoxy acetate (2,4, S-T). Toxic and App Pharm 73:492–499
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantization of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Anderson D, Yu TW, Phillips BJ, Schmerzer P (1994) The effect of various antioxidants and other modifying agents on oxygen-radical generated DNA damage inhuman lymphocytes in the comet assay. Muta Res 307:261–271
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bioch 72:248–254
Woodrow Wilson International Center for Scholars (2008) The project on emerging nanotechnologies. Consumer products. An inventory of nanotechnology-based consumer products currently on the Market http://www.nanotechproject.org/inventories/consumer.
Elzey S, Grassian VH (2010) Agglomeration, isolation and dissolution of commercially manufactured silver nanoparticles in aqueous environments. J Nanopart Res 12:1945–1958
Griffitt RJ, Luo J, Gao J, Bonzango JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanoparticles in aquatic organisms. Environ Toxic and Chem 27:1972–1978
Tola EN, Mungan MT, Uğuz AC, Naziroğlu M (2013) Intracellular Ca2+ and antioxidant values induced positive effect on fertilisation ratio and oocyte quality of granulosa cells in patients undergoing in vitro fertilization. Reprod Fertil Dev 25:746–752
Valencia A, Kochevar IE (2006) Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith-Lemli-Opitz syndrome. Free Rad Bio and Med 40:641–650
Naziroglu M (2012) Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32:134–141
Simon HU, Haj-Yehia A, Levi-Schaffer A (2000) Role of reactive oxygen species in apoptosis induction. Apoptosis 5:415–418
Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XHN (2007) In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 1:133–143
Livingstone DR, Lips F, Garcia MP, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Bio 112:265–276
Zhao M, Antunes F, Eaton JW, Brunk UT (2003) Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem 270:3778–3786
Verma RS, Mehta A, Srivastava N (2007) In vivo chlorpyrifos induced oxidative stress: attenuation by antioxidant vitamins. Pest Bioch and Phys 88:191–196
Jamil K (2001) Bioindicators and biomarkers of environmental pollution and risk assessment. (Science Publishers, Inc., Enfield (Nh). USA and Plymouth, U.K. pp 45–52
Regoli F, Hummel H, Amiard-Triquet C, Larroux C, Sukhotin A (1998) Trace metals and variations of antioxidant enzymes in Arctic bivalve populations. Archives of Environ Conta and Toxic 35:594–601
Almeida EA, Miyamoto S, Bainy ACD, Medeiros MH, Mascio P (2004) Protective effect of phosphor lipid hydroperoxide glutathione peroxidase (PHGPx) against lipid peroxidation in mussels Perna perna exposed to different metals. Mar Poll Bull 49:386–392
Roling JA, Baldwin WS (2006) Alterations in hepatic gene expression by trivalent chromium in Fundulus heteroclitus. Mar Environ Res 62:122–127
Ozkaya OM, Nazıroglu M, Barak C, Berkkanoglu M (2011) Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biol Trace Elem Res 139:1–9
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group NO (RG −1435-076).
Conflict of Interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, D. Oxidative Stress-Mediated Apoptosis and Genotoxicity Induced by Silver Nanoparticles in Freshwater Snail Lymnea luteola L.. Biol Trace Elem Res 162, 333–341 (2014). https://doi.org/10.1007/s12011-014-0158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0158-6