Abstract
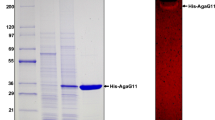
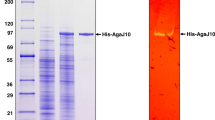
An agarase gene (agaH71) was identified from Pseudoalteromonas hodoensis, an agar utilizing marine bacterium. The nucleotide sequence revealed that AgaH71 had significant homology to glycosyl hydrolase (GH) 16 agarases. agaH71 encodes a primary translation product (32.7 kDa) of 290 amino acids, including a 21-amino-acid signal peptide. The entire AgaH71 was expressed in a fused protein with glutathione-S-transferase (GST) at its N-terminal (GST-AgaH71) in Escherichia coli. Purified GST-AgaH71 had an apparent molecular weight of 59 kDa on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which was consistent with the calculated molecular weight (58.7 kDa). Agarase activity of the purified protein was confirmed by zymogram assay. GST-AgaH71 could hydrolyze p-nitrophenyl-β-d-galactopyranoside, but not p-nitrophenyl-α-d-galactopyranoside. The optimum pH and temperature for GST-AgaH71 were 6.0 and 45 °C, respectively. GST-AgaH71 retained more than 95 and 90 % of its initial activity at 40 and 45 °C after heat treatment for 60 min, respectively. The K m and V max for agarose were 28.33 mg/ml and 88.25 U/mg, respectively. GST-AgaH71 did not require metal ions for its activity, but severe inhibition by divalent metal ions was observed. Thin-layer chromatography (TLC) analysis, mass spectrometry, and nuclear magnetic resonance (NMR) spectrometry of the GST-AgaH71 hydrolysis products revealed that GST-AgaH71 is an endo-type β-agarase that hydrolyzes agarose into predominantly neoagarotetraose and small proportions of neoagarobiose and neoagarohexaose.





Similar content being viewed by others
References
Craigie, J. (1990). Cell walls. In K. M. Cole & R. G. Sheath (Eds.), Biology of the red algae (pp. 221–258). New York: Cambridge University Press.
Hehemann, J. H., Correc, G., Barbeyron, T., Helbert, W., Czjzek, M., & Michel, G. (2010). Nature, 464, 908–912.
Morrice, L. M., McLean, M. W., Long, W. F., & Williamson, F. B. (1983). European Journal of Biochemistry, 133, 673–684.
Duckworth, M., & Yaphe, W. (1970). Journal of Chromatography, 49, 482–487.
Chiovitti, A., Bacic, A., Craik, D. J., Kraft, G. T., & Liao, M. L. (2004). Carbohydrate Research, 339, 1459–1466.
Chi, W. J., Chang, Y. K., & Hong, S. K. (2012). Applied Microbiology and Biotechnology, 94, 917–930.
Hassairi, I., Ben, A. R., Nonus, M., & Gupta, B. B. (2001). Bioresource Technology, 79, 47–51.
Duckworth, M., & Turvey, J. R. (1969). The Biochemical Journal, 113, 693–696.
Kang, N. Y., Choi, Y. L., Cho, Y. S., Kim, B. K., Jeon, B. S., Cha, J. Y., Kim, C. H., & Lee, Y. C. (2003). Biotechnology Letters, 25, 1165–1170.
Potin, P., Richard, C., Rochas, C., & Kloareg, B. (1993). European Journal of Biochemistry, 214, 599–607.
Schroeder, D. C., Jaffer, M. A., & Coyne, V. E. (2003). Microbiology, 149, 2919–2929.
Aoki, T., Araki, T., & Kitamikado, M. (1990). European Journal of Biochemistry, 187, 461–465.
Ohta, Y., Hatada, Y., Miyazaki, M., Nogi, Y., Ito, S., & Horikoshi, K. (2005). Current Microbiology, 50, 212–216.
Suzuki, H., Sawaim, Y., Suzuki, T., & Kawai, K. (2003). Journal of Bioscience and Bioengineering, 93, 456–463.
Chi, W. J., Park, J. S., Kwak, M. J., Kim, J. F., Chang, Y. K., & Hong, S. K. (2013). Journal of Microbiology and Biotechnology, 23, 1509–1518.
Hosoda, A., Sakai, M., & Kanazawa, S. (2003). Bioscience Biotechnology and Biochemistry, 67, 1048–1055.
Temuujin, U., Chi, W. J., Chang, Y. K., & Hong, S. K. (2012). Journal of Bacteriology, 194, 142–149.
Chi, W. J., Park, J. S., Kang, D. K., & Hong, S. K. (2014). Applied Biochemistry and Biotechnology. doi:10.1007/s12010-014-0958-3.
Petersen, T. N., von Brunak, S., Heijne, G., & Nielsen, H. (2011). Nature Methods, 8, 785–786.
Temuujin, U., Chi, W. J., Lee, S. Y., Chang, Y. K., & Hong, S. K. (2011). Applied Microbiology and Biotechnology, 92, 749–759.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Lineweaver, H., & Burk, D. (1934). Journal American Chemistry Society, 56, 658–666.
Henrissat, B., & Bairoch, A. (1993). Biotechnology Journal, 293, 781–788.
Oh, C., Nikapitiya, C., Lee, Y., Whang, I., Kim, S. J., Kang, D. H., & Lee, J. (2010). Journal Industry Mirobiology and Biotechnology, 37, 483–494.
Rochas, C., Lahaye, M., & Yaphe, W. (1986). Carbohydrate Research, 148, 199–207.
Liang, S. S., Chen, Y. P., Chen, Y. H., Chiu, S. H., & Liaw, L. L. (2013). Journal Applied Microbiology. doi:10.1111/jam.12389.
Pluvinage, B., Hehemann, J. H., & Boraston, A. B. (2013). The Journal of Biological Chemistry, 288, 28078–28088.
Ekborg, N., Taylor, L., Weiner, R., & Hutcheson, S. (2006). Applied and Environmental Microbiology, 72, 3396–3405.
Ohta, Y., Hatada, Y., Nogi, Y., Miyazaki, M., Akita, M., Hidaka, Y., Goda, S., Ito, S., Horikoshi, K., & Li, Z. (2004). Applied Microbiology and Biotechnology, 64, 505–514.
Dong, J., Hashikawa, S., Konishi, T., Tamaru, Y., & Araki, T. (2006). Applied and Environmental Microbiology, 72, 6399–63401.
Ma, C., Lu, X., Shi, C., Li, J., Gu, Y., Ma, Y., Chu, Y., Han, F., Gong, Q., & Yu, W. (2007). The Journal of Biological Chemistry, 282, 3747–3754.
Marchler-Bauer, A., et al. (2011). Nucleic Acids Research, 39, 225–229.
Allouch, J., Jam, M., Helbert, W., Barbeyron, T., Kloareg, B., Henrissat, B., & Czjzek, M. (2003). 278, 47171–17180.
Holmström, C., & Kjelleberg, S. (1999). FEMS Microbiology Ecology, 30, 285–293.
Vera, J., Alvarez, R., Murano, E., Slebe, J. C., & Leon, O. (1998). Applied and Environmental Microbiology, 64, 4378–4383.
Oh, C., Nikapitiya, C., Lee, Y., Whang, I., Kang, D. H., Heo, S. J., Choi, Y. U., & Lee, J. (2010). Brazilian Journal of Microbiology, 41, 876–889.
Oh, Y. H., Jung, C., & Lee, J. (2011). Journal of Microbiology and Biotechnology, 21, 818–821.
Xiao, T. F., & Kim, S. M. (2010). Marine Drugs, 8, 200–218.
Kobayashi, R., Takisada, M., Suzuki, T., Kirimura, K., & Usami, S. (1997). 61, 162–163.
Lee, D. G., Jang, M. K., Lee, O. H., Kim, N. Y., Ju, S., & Lee, S. H. (2008). Biotechnology Letters, 30, 911–918.
Chen, H. M., Zheng, L., & Yan, X. J. (2005). Food Technology and Biotechnology, 43, 29–36.
Wang, J., Jiang, X., Mou, H., & Guan, H. (2004). Journal of Applied Phycology, 16, 333–340.
Wu, S. C., Wen, T. N., & Pan, C. L. (2005). Fisheries Science, 71, 1149–1159.
Acknowledgments
This work was supported by grants PJ009536 from the Next-Generation Bio Green 21 Program, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, D.Y., Chi, WJ., Park, JS. et al. Cloning, Expression, and Biochemical Characterization of a GH16 β-Agarase AgaH71 from Pseudoalteromonas hodoensis H7. Appl Biochem Biotechnol 175, 733–747 (2015). https://doi.org/10.1007/s12010-014-1294-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1294-3