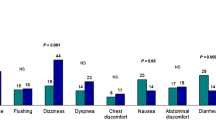
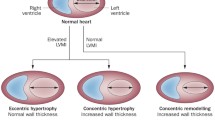
Abstract
Purpose of the Review
Cardiorenal syndrome (CRS), defined as concomitant heart and kidney disease, has been a focus of attention for nearly a decade. As more patients survive severe acute and chronic heart and kidney diseases, CRS has emerged as an “epidemic” of modern medicine. Significant advances have been made in unraveling the complex mechanisms that underlie CRS based on classification of the condition into five pathophysiologic subtypes. In types 1 and 2, acute or chronic heart disease results in renal dysfunction, while in types 3 and 4, acute or chronic kidney diseases are the inciting factors for heart disease. Type 5 CRS is defined as concomitant heart and kidney dysfunction as part of a systemic condition such as sepsis or autoimmune disease.
Recent Findings
There are ongoing efforts to better define subtypes of CRS based on historical information, clinical manifestations, laboratory data (including biomarkers), and imaging characteristics. Systematic evaluation of CRS by advanced cardiac imaging, however, has been limited in scope and mostly focused on type 4 CRS. This is in part related to lack of clinical trials applying advanced cardiac imaging in the acute setting and exclusion of patients with significant renal disease from studies of such techniques in chronic HF.
Summary
Advanced cardiac nuclear imaging is well poised for assessment of the pathophysiology of CRS by offering a myriad of molecular probes without the need for nephrotoxic contrast agents. In this review, we examine the current or potential future application of advanced cardiac imaging to evaluation of myocardial perfusion, metabolism, and innervation in patients with CRS.



Similar content being viewed by others
Abbreviations
- AKI:
-
Acute kidney injury
- BMIPP:
-
β-Methyl-p-[123I]-iodophenyl-pentadecanoic acid
- CAD:
-
Coronary artery disease
- CKD:
-
Chronic kidney disease
- CRS:
-
Cardiorenal syndrome
- ESRD:
-
End-stage renal disease
- FDG:
-
2-Deoxy-2-18Ffluoro-d-glucose
- GFR:
-
Glomerular filtration rate
- HF:
-
Heart failure
- HMR:
-
Heart-to-mediastinal ratio
- LV:
-
Left ventricle (ventricular)
- mIBG:
-
Meta-iodobenzylguanidine
- MPI:
-
Myocardial perfusion imaging
- PET:
-
Positron emission tomography
- RAAS:
-
Renin angiotensin aldosterone system
- SNS:
-
Sympathetic nervous system
- SPECT:
-
Single photon emission computed tomography
- WR:
-
Washout rate
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–80.
Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11:404–15.
Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–93.
Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–85.
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–80.
Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2018;11:e004646.
Juillière Y, Venner C, Filippetti L, Popovic B, Huttin O, Selton-Suty C. Heart failure with preserved ejection fraction: a systemic disease linked to multiple comorbidities, targeting new therapeutic options. Arch Cardiovasc Dis. 2018: in press;111:766–81.
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Perez Crespillo A, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–80.
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–30.
Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(suppl 2):S23–33.
Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. US Renal Data System 2013 annual data report. Am J Kidney Dis. 2014;63(suppl 1):e1–e420.
House AA. Management of heart failure in advancing CKD: Core Curriculum 2018. Am J Kidney Dis. 2018;72:284–95.
House AA, Anand I, Bellomo R, Cruz D, Bobek I, Anker SD, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1416–20.
Brisco MA, Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep. 2014;11:485–99.
• Roncoa C, Di Lullob L. Cardiorenal Syndrome in western countries: epidemiology, diagnosis and management approaches. Kidney Dis (Basel). 2017;2:151–63 This is a comprehensive review of cardiorenal syndrome with examples of each type, epidemiology of the condition and discussion of diagnosis and management.
Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ, ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–580.
• Mavrakanas TA, Khattak A, Singh K, Charytan DM. Epidemiology and natural history of the cardiorenal syndromes in a cohort with echocardiography. Clin J Am Soc Nephrol. 2017;12:1624–33 This is a retrospective cohort study of adults who underwent transthoracic echocardiography between 2004 and 2014 used to derive the prevalence and natural history of various types of cardiorenal syndrome .
• Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the acute heart failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19:821–36 This is an authoritative and informative review of the current understanding of the presentation and management of concurrent organ impairment, including kidney dysfunction, in acute heart failure.
Gnanaraj J, von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio-renal syndrome. Kidney Int. 2013;83:384–91.
Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, et al. Cardio-renal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74.
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8.
Anand IS, Greenberg BH, Katra RP, Fogoros RN, Libbus I, Katra RP, et al. Design of the Multi-Sensor Monitoring in Congestive HF (MUSIC) study: prospective trial to assess the utility of continuous wireless physiologic monitoring in heart failure. J Card Fail. 2011;17:11–6.
•• Puzzovivo A, Monitillo F, Guida P, Leone M, Rizzo C, Grande D, et al. Renal venous pattern: a new parameter for predicting prognosis in heart failure outpatients. J Cardiovasc Dev Dis. 2018;5:E52 This is an insightful study that demonstrates the significance of renal congestion in predicting progression of heart failure and characterization of cardiorenal syndrome through the eyes of renal vein Doppler pattern.
Cruz DN, Fard A, Clementi A, Ronco C, Maisel A. Role of biomarkers in the diagnosis and management of cardio-renal syndromes. Semin Nephrol. 2012;32:79–92.
Shirani J, Singh A, Agrawal S, Dilsizian V. Cardiac molecular imaging to track left ventricular remodeling in heart failure. J Nucl Cardiol. 2017;24:574–90.
Shirani J, Narula J, Eckelman WC, Narula N, Dilsizian V. Early imaging in heart failure: exploring novel molecular targets. J Nucl Cardiol. 2007;14:100–10.
Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–62.
Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–9.
Zhang DY, Anderson AS. The sympathetic nervous system and heart failure. Cardiol Clin. 2014;32:33–45.
Sayer G, Bhat G. The renin-angiotensin-aldosterone system and heart failure. Cardiol Clin. 2014;32:21–32.
McCullough PA, Kellum JA, Mehta RL, Murray PT, Ronco C. ADQI consensus on AKI biomarkers and cardiorenal syndromes. Contrib Nephrol. 2013;182:82–98.
Preeti J, Alexandre M, Pupalan I, Merlin TC, Claudio R. Chronic heart failure and comorbid renal dysfunction—a focus on type 2 cardiorenal syndrome. Curr Cardiol Rev. 2016;12:186–94.
•• Fu S, Zhao S, Ye P, Luo L. Biomarkers in cardiorenal syndromes. Biomed Res Int. 2018:9617363 This is a comprehensive review of established and possibly useful biomarkers that are predictive of heart failure and renal diseases with a potential for identifying cardiac dysfunction in renal diseases and renal injury in heart failure.
Clementi A, Virzì GM, Brocca A, de Cal M, Pastori S, Clementi M, et al. Advances in the pathogenesis of cardiorenal syndrome type 3. Oxidative Med Cell Longev. 2015;2015:148082.
Petrucci I, Clementi A, Sessa C, Torrisi I, Meola M. Ultrasound and color Doppler applications in chronic kidney disease. J Nephrol. 2018;31:863–79.
Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G; European Renal and Cardiovascular Medicine (EURECA-m) Working Group of the European Renal Association - European Dialysis Transplantation Association (ERA-EDTA). The systemic nature of CKD. Nat Rev Nephrol 2017;13:344–358.
Roberts WC, Taylor MA, Shirani J. Cardiac findings at necropsy in patients with chronic kidney disease maintained on chronic hemodialysis. Medicine (Baltimore). 2012;91:165–78.
Bhatti NK, Karimi GK, Paz Y, Nazif T, Moses JW, Leon MB, et al. Diagnosis and management of cardiovascular disease in advanced and end-stage renal disease. J Am Heart Assoc. 2016;5:e003648.
Stromp TA, Spear TJ, Holtkamp RM, Andres KN, Kaine JC, Alghuraibawi WH, et al. Quantitative gadolinium-free cardiac fibrosis imaging in end stage renal disease patients reveals a longitudinal correlation with structural and functional decline. Sci Rep. 2018;8:16972.
Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C, McCullough PA, et al. Cardiorenal syndrome type 5: clinical presentation, pathophysiology and management strategies from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol. 2013;182:174–94.
Lekawanvijit S. Cardiotoxicity of uremic toxins: a driver of cardiorenal syndrome. Toxins (Basel). 2018;10:E352.
Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, et al. Progression of coronary artery calcification in predialysis patients. Am J Nephrol. 2007;27:152–8.
Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218–23.
Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605.
•• Moody WE, Lin EL, Stoodley M, McNulty D, Thomson LE, Berman DS, et al. Prognostic utility of calcium scoring as an adjunct to stress myocardial perfusion scintigraphy in end-stage renal disease. Am J Cardiol. 2016;117:1387–96 This study showed that coronary artery calcium score does not have an incremental value to myocardial perfusion imaging for prediction of adverse cardiac outcomes in renal transplant candidates.
Wang LW, Fahim MA, Hayen A, Mitchell RL, Lord SW, Baines LA, et al. Cardiac testing for coronary artery disease in potential kidney transplant recipients: a systematic review of test accuracy studies. Am J Kidney Dis. 2011;57:476–87.
Herzog CA, Natwick T, Li S, Charytan DM. Comparative utilization and temporal trends in cardiac stress testing in U.S. medicare beneficiaries with and without chronic kidney disease. JACC Cardiovasc Imaging 2018 [Epub ahead of print].
Gewirtz H, Dilsizian V. Integration of quantitative PET absolute myocardial blood flow in the clinical management of coronary artery disease. Circulation. 2016;133:2180–96.
Dilsizian V, Chandrashekhar Y, Narula J. Quantitative PET myocardial blood flow: trust, but verify. JACC Cardiovasc Imaging. 2017;10:609–10.
Schindler TH, Dilsizian V. PET-determined hyperemic myocardial blood flow: further progress to clinical application. J Am Coll Cardiol. 2014;64:1476–8.
Dilsizian V. Transition from SPECT to PET myocardial perfusion imaging: a desirable change in nuclear cardiology to approach perfection. J Nucl Cardiol. 2016;23:337–8.
Dilsizian V, Bacharach SL, Beanlands SR, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–226.
Golzar Y, Doukky R. Stress SPECT myocardial perfusion imaging in end-stage renal disease. Curr Cardiovasc Imaging Rep 2017;10:Epub 2017 Mar 18.
•• Paz Y, Morgenstern R, Weinberg R, Chiles M, Bhatti N, Ali Z, et al. Relation of coronary flow reserve to other findings on positron emission tomography myocardial perfusion imaging and left heart catheterization in patients with end-stage renal disease being evaluated for kidney transplant. Am J Cardiol. 2017;120:1909–12 This prospective study demonstrated for the first time that abnormal coronary flow reserve as assessed by positron emission tomography myocardial perfusion imaging is frequently present in patients with end-stage renal disease and is independent of perfusion defects or obstructive coronary artery disease.
Fukushima K, Javadi MS, Higuchi T, Bravo PE, Chien D, Lautamäki R, et al. Impaired global myocardial flow dynamics despite normal left ventricular function and regional perfusion in chronic kidney disease: a quantitative analysis of clinical 82Rb PET/CT studies. J Nucl Med. 2012;53:887–8893.
Lodge MA, Braess H, Mahmood F, Suh JD, Englar N, Bacharach SL, et al. Developments in nuclear cardiology: transition from single photon emission computed tomography to positron emission tomography-computed tomography. J Invasive Cardiol. 2005;17:491–6.
Dilsizian V, Taillefer R. Journey in evolution of nuclear cardiology: will there be another quantum leap with the F-18 labeled myocardial perfusion tracers? JACC Cardiovasc Imaging. 2012;5:1269–84.
Dilsizian V. Metabolic adaptation to myocardial ischemia: the role of fatty acid imaging. J Nucl Cardiol. 2007;14(Suppl 3):S97–9.
Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–8.
Dilsizian V, Arrighi JA, Diodati JG, Quyyumi AA, Bacharach SL, Alavi K, et al. Myocardial viability in patients with chronic coronary artery disease: comparison of 99mTc-sestamibi with thallium reinjection and 18F-fluorodeoxyglucose. Circulation. 1994;89:578–87.
Srinivasan G, Kitsiou AN, Bacharach SL, Bartlett ML, Miller-Davis C, Dilsizian V. 18F-fluorodeoxyglucose single photon emission computed tomography: can it replace PET and thallium SPECT for the assessment of myocardial viability? Circulation. 1998;97:843–50.
Kitsiou AN, Bacharach SL, Bartlett ML, Srinivasan G, Summers RM, Quyyumi AA, et al. 13N-ammonia myocardial blood flow and uptake: relation to functional outcome of asynergic regions after revascularization. J Am Coll Cardiol. 1999;33:678–86.
Osterholt M, Sen S, Dilsizian V, Taegtmeyer H. Targeted metabolic imaging to improve the management of heart disease. JACC Cardiovasc Imaging. 2012;5:214–26.
•• Sengupta PP, Kramer CM, Narula J, Dilsizian V. The potential of clinical phenotyping of heart failure with imaging biomarkers for guiding therapies: a focused update. JACC Cardiovasc Imaging. 2017;10:1056–71 This review presents potential application of advanced cardiac imaging in assessment of heart failure phenotype and as a guide to selecting specific and evidence-based treatment.
Gewirtz H, Dilsizian V. Myocardial viability: survival mechanisms and molecular imaging targets in acute and chronic ischemia. Circ Res. 2017;120:1197–212.
•• Fink JC, Lodge MA, Smith MF, Hinduja A, Brown J, Dinits-Pensy MY, et al. Pre-clinical myocardial metabolic alterations in chronic kidney disease. Cardiology. 2010;116:160–7 This original prospective study showed for the first time that a significant inverse correlation existed between myocardial glucose utilization and severity of kidney dysfunction that could not be explained by demographic factors or cardiac workload.
Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018–22.
McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26.
Dasselaar JJ, Slart RH, Knip M, Pruim J, Tio RA, McIntyre CW, et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2009;24:604–10.
Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, et al. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol. 2012;7:1615–23.
Breidthardt T, Burton JO, Odudu A, Eldehni MT, Jefferies HJ, McIntyre CW. Troponin T for the detection of dialysis-induced myocardial stunning in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:1285–92.
Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–20.
Dilsizian V, Bateman TM, Bergmann SR, Des Prez R, Magram M, Goodbody AE, et al. Metabolic imaging with β-methyl-ρ-[123I]-iodophenyl-pentadecanoic acid (BMIPP) identifies ischemic memory following demand ischemia. Circulation. 2005;112(14):2169–74.
Taegtmeyer H, Dilsizian V. Imaging myocardial metabolism and ischemic memory. Nat Clin Pract Card. 2008;5:S42–8.
Dilsizian V. FDG uptake as a surrogate marker for antecedent ischemia. J Nucl Med. 2008;49:1909–11.
Dilsizian V, Fink JC. Deleterious effect of altered myocardial fatty acid metabolism in kidney disease. J Am Coll Cardiol. 2008;51:146–8.
Nishimura M, Hashimoto T, Kobayashi H, Fukuda T, Okino K, Yamamoto N, et al. Myocardial scintigraphy using a fatty acid analogue detects coronary artery disease in hemodialysis patients. Kidney Int. 2004;66:811–9.
Nishimura M, Murase M, Hashimoto T, Kobayashi H, Yamazaki S, Imai R, et al. Insulin resistance and impaired myocardial fatty acid metabolism in dialysis patients with normal coronary arteries. Kidney Int. 2006;69:553–9.
Nishimura M, Tsukamoto K, Hasebe N, Tamaki N, Kikuchi K, Ono T. Prediction of cardiac death in hemodialysis patients by myocardial fatty acid imaging. J Am Coll Cardiol. 2008;51:139–45.
Nishimura M, Tokoro T, Nishida M, Hashimoto T, Kobayashi H, Yamazaki S, et al. Myocardial fatty acid imaging identifies a group of hemodialysis patients at high risk for cardiac death after coronary revascularization. Kidney Int. 2008;74:513–20.
Moroi M, Tamaki N, Nishimura M, Haze K, Nishimura T, Kusano E, et al. Association between abnormal myocardial fatty acid metabolism and cardiac-derived death among patients undergoing hemodialysis: results from a cohort study in Japan. Am J Kidney Dis. 2013;61:466–75.
Nishimura M, Hashimoto T, Tamaki N, Kobayashi H, Ono T. Focal impairment in myocardial fatty acid imaging in the left anterior descending artery area, a strong predictor for cardiac death in hemodialysis patients without obstructive coronary artery disease. Eur J Nucl Med Mol Imaging. 2015;42:1612–21.
Eckelman WC, Dilsizian V. Chemistry and biology of radiotracers that target changes in sympathetic and parasympathetic nervous system in heart disease. J Nucl Med. 2015;56:7S–10S.
Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev. 2011;16:101–7.
Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–22.
•• Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21 This is a pivotal study that lead to Federal Drug Administration’s approval of meta-iodobenzylguanidine imaging for risk stratification of patients with heart failure and reduced systolic function.
Dilsizian V, Chandrashekhar Y, Narula J. Introduction of new tests: low are the mountains, high the expectations. JACC Cardiovasc Imaging. 2010;3:117–9.
• Badarin FJ, Wimmer AP, Kennedy KF, Jacobson AF, Bateman TM. The utility of ADMIRE-HF risk score in predicting serious arrhythmic events in heart failure patients: Incremental prognostic benefit of cardiac 123I-mIBG scintigraphy. J Nucl Cardiol. 2014;21:756–62 This was a post-hoc evaluation of data from ADMIRE-HF that showed the utility of meta-iodobenzylguanidine imaging in predicting serious arrhythmic events in patients with heart failure and reduced systolic function.
Agostini D, Belin A, Amar MH, Darlas Y, Hamon M, Grollier G, et al. Improvement of cardiac neuronal function after carvedilol treatment in dilated cardiomyopathy: a 123I-MIBG scintigraphic study. J Nucl Med. 2000;41:845–51.
Gerson MC, Craft LL, McGuire N, Suresh DP, Abraham WT, Wagoner LE. Carvedilol improves left ventricular function in heart failure patients with idiopathic dilated cardiomyopathy and a wide range of sympathetic nervous system function as measured by iodine 123 metaiodobenzylguanidine. J Nucl Cardiol. 2002;9:608–15.
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Addition of valsartan to an angiotensin-converting enzyme inhibitor improves cardiac sympathetic nerve activity and left ventricular function in patients with congestive heart failure. J Nucl Med. 2003;44:884–90.
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med. 2002;43:1279–85.
Cha YM, Oh J, Miyazaki C, Hayes DL, Rea RF, Shen WK, et al. Cardiac resynchronization therapy upregulates cardiac autonomic control. J Cardiovasc Electrophysiol. 2008;19:1045–52.
Drakos SG, Athanasoulis T, Malliaras KG, Terrovitis JV, Diakos N, Koudoumas D, et al. Myocardial sympathetic innervation and long-term left ventricular mechanical unloading. JACC Cardiovasc Imaging. 2010;3:64–70.
Nishioka SA, Martinelli Filho M, Brandão SC, Giorgi MC, Vieira ML, Costa R, et al. Cardiac sympathetic activity pre and post resynchronization therapy evaluated by 123I-MIBG myocardial scintigraphy. J Nucl Cardiol. 2007;14:852–9.
Nakajima K, Verschure DO, Okuda K, Verberne HJ. Standardization of (123)I-meta-iodobenzylguanidine myocardial sympathetic activity imaging: phantom calibration and clinical applications. Clin Transl Imaging. 2017;5(3):255–63.
Kurata C, Wakabayashi Y, Shouda S, Okayama K, Yamamoto T, Ishikawa A, et al. Enhanced cardiac clearance of iodine-123-MIBG in chronic renal failure. J Nucl Med. 1995;36:2037–43.
Kurata C, Uehara A, Sugi T, Ishikawa A, Fujita K, Yonemura K, et al. Cardiac autonomic neuropathy in patients with chronic renal failure on hemodialysis. Nephron. 2000;84:312–9.
Chrapko BE, Jaroszyński AJ, Głowniak A, Bednarek-Skublewska A, Załuska W, Ksiażek A. Iodine-123 metaiodobenzylguanidine myocardial imaging in haemodialysed patients asymptomatic for coronary artery disease: a preliminary report. Nucl Med Commun. 2011;32:515–21.
Noordzij W, Özyilmaz A, Glaudemans AWJM, Tio RA, Goet ER, Franssen CFM, et al. Investigation into cardiac sympathetic innervation during the commencement of haemodialysis in patients with chronic kidney disease. Eur Radiol Exp. 2017;1:24.
Furuhashi T, Moroi M. Importance of renal function on prognostic value of cardiac iodine-123 metaiodobenzylguanidine scintigraphy. Ann Nucl Med. 2007;21:57–63.
Verschure DO, Somsen GA, van Eck-Smit BL, Verberne HJ. Renal function in relation to cardiac (123)I-MIBG scintigraphy in patients with chronic heart failure. Int J Mol Imaging. 2012;2012:434790.
Doi T, Nakata T, Hashimoto A, Yuda S, Wakabayashi T, Kouzu H, et al. Cardiac mortality assessment improved by evaluation of cardiac sympathetic nerve activity in combination with hemoglobin and kidney function in chronic heart failure patients. J Nucl Med. 2012;53:731–40.
Kouzu H, Doi T, Kawamukai M, Nishida J, Mochizuki A, Muranaka A, et al. Prognostic value of cardiac sympathetic imaging with metaiodobenzylguanidine for the prediction of mortality in patients with cardiorenal syndrome. Circulation. 2018;124:A9393.
Matsunari I, Aoki H, Nomura Y, Takeda N, Chen WP, Taki J, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging. 2010;3:595–603.
Dilsizian V, Eckelman WC. Myocardial blood flow and innervation measures from a single scan: an appealing concept but a challenging paradigm. J Nucl Med. 2015 Nov;56(11):1645–6.
Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880–9.
Chen W, Dilsizian V. Molecular imaging of amyloidosis: will the heart be the next target after the brain? Curr Cardiol Rep. 2012;14(2):226–33.
Chen W, Ton VK, Dilsizian V. Clinical phenotyping of transthyretin cardiac amyloidosis with bone seeking radiotracers in heart failure with preserved ejection fraction. Curr Cardiol Rep. 2018;20(4):23.
Pilebro B, Arvidsson S, Lindqvist P, Sundström T, Westermark P, Antoni G, et al. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J Nucl Cardiol. 2018;25:240–8.
Pilebro B, Arvidsson S, Lindqvist P, Sundström T, Westermark P, Antoni G, et al. (123)I-Labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging. 2012;39:1609–17.
Coutinho MC, Cortez-Dias N, Cantinho G, Conceição I, Oliveira A, Bordalo e Sá A, et al. Reduced myocardial 123-iodine metaiodobenzylguanidine uptake: a prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging. 2013;6(5):627–36.
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53(2):241–8.
Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867.
Tomberli B, Cecchi F, Sciagrà R, Berti V, Lisi F, Torricelli F, et al. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson-Fabry disease. Eur J Heart Fail. 2013;15:1363–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Shirani, J., Meera, S. & Dilsizian, V. The Cardiorenal Axis: Myocardial Perfusion, Metabolism, and Innervation. Curr Cardiol Rep 21, 60 (2019). https://doi.org/10.1007/s11886-019-1147-3
Published:
DOI: https://doi.org/10.1007/s11886-019-1147-3