Abstract
Background
DPD scintigraphy has been advocated for imaging cardiac amyloid in ATTR amyloidosis. PET utilizing 11C-Pittsburgh compound B (PIB) is the gold standard for imaging brain amyloid in Alzheimer’s disease. PIB was recently shown to identify cardiac amyloidosis in both AL and ATTR amyloidosis. In the ATTR population, two types of amyloid fibrils exist, one containing fragmented and full-length TTR (type A) and the other only full-length TTR (type B). The aim of this study was to further evaluate PIB-PET in patients with hereditary ATTR amyloidosis.
Methods
Ten patients with biopsy-proven V30M ATTR amyloidosis and discrete or no signs of cardiac involvement were included. Patients were grouped according to TTR-fragmentation. All underwent DPD scintigraphy, echocardiography, and PIB-PET. A left ventricular PIB-retention index (PIB-RI) was established and compared to five normal volunteers.
Results
PIB-RI was increased in all patients (P < 0.001), but was significantly higher in type B than in type A (0.129 ± 0.041 vs 0.040 ± 0.006 min−1, P = 0.009). Cardiac DPD uptake was elevated in group A and absent in group B.
Conclusion
PIB-PET, in contrast to DPD scintigraphy, has the potential to specifically identify cardiac amyloid depositions irrespective of amyloid fibril composition. The heart appears to be a target organ for amyloid deposition in ATTR amyloidosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Transthyretin (TTR) amyloidosis (ATTR) is a disease where wild-type or mutant TTR assembles into amyloid. It is a systemic disease that affects mainly the heart and nervous system.1 Over 100 different disease-causing mutations in the TTR gene have been identified.2 There is a pronounced variation in the presentation of the disease, where neuropathy or cardiomyopathy may dominate, but most mutations have symptoms from both the heart and nervous system. In addition, being a systemic disease, gastrointestinal, renal, visual, and other organ systems can be involved. These phenotypic variations are in part tied to the disease-causing mutation and probably age at onset, but also to others as yet unknown factors.3
Proteolysis and fragmentation of TTR appear to play a central role in the development of TTR cardiomyopathy.4,5 Among patients with ATTR V30M amyloidosis, the most common TTR mutation in Europe, two phenotypes are noted: one characterized by late-onset disease (50 ≥ years of age) and with both cardiac and neurological manifestations, and the other by an early-onset disease (<50 years of age) and with mainly neuropathic manifestation.6 These phenotypes have been tied to differences in amyloid fibril composition.4,5 Amyloid from patients with late-onset disease contains both full-length and N-terminal TTR fragments starting at position 46-52 (Type A fibril pattern), whereas amyloid of early-onset disease patients typically contains full-length TTR only (Type B fibril pattern).7 The type B fibril pattern has so far only been detected in the V30M population and in patients carrying the rare Y144C mutation. Amyloid deposits from patients with other mutations or wild-type ATTR amyloidosis have all contained TTR fragments.8 Until recently, the only available treatment for ATTR amyloidosis was liver transplantation. However, subsequent analysis has shown that patients with type B fibrils, generally early-onset V30M patients had a substantial better outcome compared with type A patients, i.e., generally late-onset V30M and non-V30M patients.9 If amyloid fibril composition has any impact on the efficacy of pharmacological treatment has so far not been investigated.
There are currently several diagnostic methods available for detection of ATTR cardiomyopathy. Despite the emergence of other modalities, echocardiography remains the basis for diagnosis and follow-up. For the purpose of early detection of ATTR cardiomyopathy, 99mtechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) scintigraphy is by many considered the gold standard.10 The exact mechanisms by which DPD interacts with cardiac amyloid deposits are not known, neither is it clear why some patients with AL amyloidosis display DPD uptake and others do not.
A class of novel tracers, originating from thioflavin T, has been developed for specific amyloid imaging of the brain in Alzheimer’s disease using the positron emission tomography (PET) and is currently regarded as the non-invasive gold standard for amyloid detection in the brain.11 Carbon-11-labeled Pittsburgh compound B (11C-PIB), the original PET amyloid tracer, should in theory bind to beta-pleated sheets in general, even outside the brain. In a small pilot study 11C-PIB PET showed a high affinity for cardiac amyloid deposits12 in both light chain amyloidosis (AL) and ATTR amyloidosis. This was reproduced by Lee et al in 22 patients with AL amyloidosis.13 Another related tracer, 18F-Florbetapir, has similarly been proven to identify cardiac amyloid deposits with high diagnostic sensitivity,14 and specific binding to cardiac amyloid has been shown.15 No previous study has investigated amyloid PET-tracer uptake in ATTR amyloidosis patients with known amyloid precursor protein; before further evaluation can be done in larger prospective studies, the impact of amyloid fibril composition on tracer retention needs to be investigated.
The aim of this study was to evaluate the impact of amyloid fibril composition on PET/CT using 11C-PIB in biopsy-proven ATTRV30M amyloidosis patients, and to compare the outcome with that obtained by DPD scintigraphy.
Material and Method
Patients Selection
Ten patients with genetically and histopathologically diagnosed neuropathic ATTRV30M amyloidosis were included in the study. The amyloid fibril type, i.e., type A or B, had been determined in fat pad biopsy for all patients. Biopsies were analyzed regarding fibril type according to a standardized Western blot method.7 We chose to include five patients with type A and five patients with type B fibrils in the study. In addition, to enable investigation of the impact of amyloid fibril type, the patients selected had similar echocardiographic findings displaying little or mild signs of cardiomyopathy, and they were of comparable age and had similar disease duration
Healthy Volunteers
Five healthy subjects without signs or symptoms of cardiac disease were included (3 males, 2 females, age range 54-75). This cohort was also used as healthy controls in a previously published study.12
Patient Characteristics
Patients were interviewed and their files were reviewed for information concerning symptoms, disease duration, cardiovascular comorbidities (hypertension, known vascular disease, renal insufficiency), and previous and current treatment for ATTR amyloidosis. The cardiac biomarkers troponin T and NT-proBNP were analyzed at the clinical chemistry laboratory of Umeå University Hospital, according to routine practice using a Cobas 8000 machine and troponin T hs STAT and proBNP II STAT reagents (Roche Scandinavia, Bromma, Sweden).
PET/CT Procedure
After low-dose CT scanning, a 25-minutes dynamic emission scan of the heart was started simultaneously with the intravenous bolus injection of 11C-PIB (6 MBq/kg) using a Discovery ST PET/CT scanner (GE Healthcare). Imaging was performed in 3-dimensional mode, yielding 47 transaxial images. All appropriate corrections for scanner normalization, dead time, decay, scatter, randoms, and attenuation were applied. Images were reconstructed into time frames of 29 frames (12 × 5, 6 × 10, 4 × 30, 2 × 60, 2 × 120, and 3 × 300 s) using ordered-subset expectation maximization (2 iterations, 21 subsets), with the application of a 5-mm Gaussian filter. Images consisted of 128 × 128 voxels, with dimensions of 2.34 × 2.34 × 3.27 mm, and a spatial resolution of approximately 7 mm.
11C-PIB PET Image Analysis
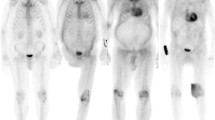
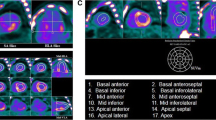
The analysis was performed using a dedicated software (Carimas V2.6, Turku, Finland) as previously described.16 Briefly, the left ventricular wall was outlined semiautomatically and the regional radioactivity from 10 to 15 minutes after injection was calculated. A small volume of interest was positioned centrally in the left ventricular (LV) cavity, and a time-activity curve from 0 to 15 minutes after injection was integrated to calculate the area under the curve (AUC). An 11C-PIB retention index (RI) was then calculated by dividing LV wall activity by the AUC and global LV RI values. Summed PET images from 15 to 25 minutes after injection were fused with CT to visually confirm cardiac 11C-PIB uptake (Figure 1). The RI was presented as a parametric polar map (see Figure 2)
Representative polar maps plots of the 11C-PIB retention index in the left ventricular wall of a healthy control subject (HC), a patient with both full-length and fragmented amyloid fibrils (Type A), and a patient with full-length fibrils only (Type B). The images were calibrated to the same absolute color scale with a maximum of 0.1 mL·min−1·mL−1
Echocardiography
Patients were investigated with two-dimensional and Doppler echocardiography using Vivid E9 (GE Medical systems, Horten, Norway),17,18 and offline analysis was carried out using Echopac PC version 113 (GE Healthcare). End-diastolic interventricular septum (IVS) thickness (IVSD) was measured from parasternal long axis view. From apical four-chamber view, left atrium (LA) volume was measured in end-systole using biplane area-length method and left ventricular ejection fraction (LVEF) was calculated using manual biplane Simpson model. Speckle tracking analysis was performed using automated function imaging (AFI) for calculation of LV global longitudinal strain from apical four-, three-, and two-chamber views. The mitral annulus and apex were manually outlined by the use of three index points followed by automatic definition of the endocardial border, mid-layer, and epicardial border in each frame throughout the cardiac cycle.19 Reduced LV global strain was defined as a value ≥16 %.20
DPD Scintigraphy
All patients were scanned using a hybrid single-photon emission computerized tomography (SPECT)-CT gamma camera (Infinia Hawkeye, GE) with a Low Energy High Resolution collimator after intravenous injection of 740 MBq of DPD. Whole body planar images were acquired 5 minutes and 3 hours postinjection in a 256 × 1024 matrix followed by a cardiac SPECT-CT with a low-dose, non-contrast CT scan. SPECT acquisition was made with a 128 × 128 matrix size in 30 projections followed by an iterative (OSEM, 3 iterations, 10 subsets) reconstruction with CT-based attenuation correction. SPECT-CT reconstruction and image fusion were performed on a GE Xeleris workstation. The CT volume data were reconstructed into 5 mm slice thickness.
Visual scoring of cardiac retention was made according to a suggested method11,12 (i.e., score 0, absent cardiac uptake and normal bone uptake; score 1, mild cardiac uptake, inferior to bone uptake; score 2, moderate cardiac uptake accompanied by attenuated bone uptake; score 3, strong cardiac uptake with mild/absent bone uptake). A score of 1, 2, or 3 was considered as DPD positive. Two experienced specialists in nuclear medicine independently performed image analysis. There were no disagreements.
Endomyocardial Biopsy (EMB)
To validate the PIB-PET findings in patients with type B amyloid fibril composition, these patients were asked to volunteer to cardiac tissue sampling. One patient had undergone EMB previously and only one of the remaining four agreed to the procedure. Biopsies were taken from the right chamber according to the standard protocol.
Ethics
The study was conducted according to the Helsinki declaration. All patients had given their written consent. The central ethical review board in Umeå approved all study procedures.
Statistics
11C-PIB RI was compared among Group A and B and Healthy Volunteers using a Friedman test. The group means were compared using Mann–Whitney U tests. Statistical analyses were performed using Graphpad Prism 5.
Results
Patient Characteristics
The patient characteristics are summarized in Table 1. There were no significant differences in disease duration, prevalence of hypertension, or age between group A and group B. Three patients with type B fibrils had undergone liver transplant prior to investigation—one 16 years prior to, and the other two during the year before the investigation. Three patients were on investigational drug treatment with siRNA (Patirisan, Alnylam). One patient in the type B group had iatrogenic ATTR amyloidosis, having received a liver from a patient with ATTR amyloidosis as part of a domino transplant procedure.21 Two patients with type B fibrils had cardiac pacemakers -one due to high level AV-block and the other SA-block; these two patients also hade renal insufficiency and elevated NT-proBNP (Table 2). In one patient with type B fibril composition, endomyocardial biopsy had been performed, which was amyloid positive. None of the patients had undergone cardiac surgery or had known coronary disease. All imaging was performed within one year for each patient.
DPD Scintigraphy
None of the patients with type B amyloid fibril composition had DPD uptake. Of the patients with type A fibrils, four had grade 2 and one had grade 3 DPD uptake (Table 2; Figure 1).
Echocardiography
No significant differences were observed between the two patient groups regarding LV dimensions and wall thickness or LA size (Table 3). No patient had increased amounts of pericardial effusion. Seven patients had increased LV wall thickness (wall thickness >12 mm). Patients from both subgroups had mainly LV wall thickening localized to the IVS. One patient (number 9) had predominant apical thickening. In two patients, increased LV thickness was localized to the basal part of IVS. Three patients (no 6, 7 and 9) had reduced LV global longitudinal strain. Reduced strain was primarily seen in segments with more pronounced wall thickening.
11C-PIB
All patients, regardless of fibril type, had increased myocardial PIB uptake compared to healthy volunteers (P < 0.001) (Figure 1). When measuring 11C-PIB RI, the 11C-PIB uptake was three times lower in patients with type A fibrils compared to those with type B fibrils (Table 3, mean 0.040 ± 0.006 vs 0.129 ± 0.041, P = 0.001). The pattern of 11C-PIB uptake differed between the two groups. In the type A group, the uptake was relatively homogenous in all patients, but in the type B group, uptake was patchy and unevenly distributed throughout the myocardium in four out of the five patients (Figure 2).
Endomyocardial Biopsies
One patient (no 9) in the study population had seven years prior to the study undergone an EMB, which was positive for amyloid. Only one (no 3) of the remaining four patients with type B amyloid agreed to the procedure. In that patient the biopsy showed rich subendocardial as well as myocardial amyloid deposits, appearing in part intracellularly (Figure 3).
Endomyocardial biopsy stained with Congo red and visualized in polarized light between crossed polars (A). A considerable amount of amyloid is present subendocardially (arrows) but also between cardiomyocytes. In B, amyloid is immunolabeled with an antibody against TTR. Bar in A 50 µm and in B 20 µm
Discussion
We have previously shown that ATTR Val30Met fibrils are of two different variants of which one consists of full-length TTR (type B) and the other of a mixture of full-length TTR, and TTR fragments starting around position 50 (type A).7 Type A and type B deposits have different histological appearance that includes fibril morphology, tinctorial properties with Congo red as well as distribution in the cardiac muscle. It is therefore interesting that 11C-PIB seems to bind much stronger to type B than to type A fibrils. Therefore, the quantitative aspects of 11C-PIB binding to ATTR amyloid might depend on fibril type, rather than amyloid burden. In addition to different binding properties of type A and B fibrils, it is not possible to exclude factors that would minimize binding access such as fibrotic encapsulation of amyloid deposits and reduced capillary patency in the proximity of amyloid.
This reasoning holds true also for DPD. Absent DPD uptake in type B hearts suggests that DPD either does not have a binding site on type B fibrils or binding occurs related to a secondary phenomenon that is not present in cardiac type B. The exact binding site for DPD is not known, but since DPD is widely used for imaging of bone mineralization, the most likely binding is associated with microcalcification. A case study using 18F-sodium fluoride and PET, which is a specific marker of cardiac microcalcification,22 was recently shown to identify TTR amyloid,23 thus supporting that microcalcification occurs in TTR. The current study indicates that not only radiolabeled ligands but also therapeutic drugs can be expected to exert different binding properties in the context of amyloid fibril subtype.
A positive DPD scintigraphy is strongly associated with future development of amyloid cardiomyopathy. DPD scintigraphy in our study was positive only in patients with type A fibrils. 11C-PIB retention was three times higher in patients with type B fibril, compared to the group with type A fibrils. This suggests that the magnitude of 11C-PIB retention does not necessarily imply cardiomyopatic development. Echocardiographic abnormalities in patients with type B fibrils might in part be ascribed by other factors. In our study, 2/3 type B patients with increased LV wall thickness had long standing renal insufficiency and hypertension. Our study further suggests that the level and pattern of 11C-PIB and DPD retention are strongly associated with fibrillar type, indicating that the combination of 11C-PIB PET and DPD scintigraphy might be used for accurate subclassification of the ATTR fibril type.
The 11C-PIB retention that we measured was compared to a small group of healthy volunteers with no signs or history of cardiac pathology. Although these individuals most likely have no cardiac amyloid depositions, the PIB RI was not equal to zero. This is related to the low resolution of the PET scanner (5-6 mm), causing spill-over of activity from the blood pool into the myocardial region of interest. Visually, as shown in Figure 1, the absence of retention in the healthy volunteers is obvious in comparison to all patients. False positive results have so far not been reported, but larger cohorts are still needed to calculate the true sensitivity and specificity of 11C-PIB in cardiac amyloidosis.
The presence of amyloid depositions in endomyocardial biopsies from patients with positive DPD scintigraphy has been used to secure the diagnosis of cardiac amyloidosis.24 Biopsy proof of absence of amyloid deposition in cardiac tissues from patients with negative DPD scintigraphy has not been provided. It is, however, well known that amyloid deposition can be detected in tissues from well-functioning and macroscopically normal appearing organs such as the GI-tract or adipose tissue. 11C-PIB PET has been used extensively in detection of brain amyloid since 2002. The incidental finding of increased 11C-PIB uptake in cortical brain areas of clinically non-demented subjects initially caused concern about the specificity of 11C-PIB for diagnosis of Alzheimer’s disease. However, the majority of these subjects developed clinically detectable dementia within a follow-up period of a few years. The finding of abnormal PIB uptake in the brain of patients with mild cognitive dysfunction is now considered prodromal Alzheimer’s disease and is a validated inclusion criteria in drug trials.25 Thus, our findings of positive 11C-PIBzz PET examination of echocardiographically normal appearing hearts are not unexpected and could prove clinically valuable. Early detection of the disease before irreversible cardiac or neurological damage has occurred is important and the long-term outcome of novel pharmacological therapies will be depending on the severity of organ damage at the time of diagnosis. Sampling errors using biopsies from any organ are not uncommon and direct cardiac biopsies are often avoided until the clinical presentation is manifest. Based on the preliminary results of our study, cardiac 11C-PIB PET might be a sensitive marker of early systemic ATTR amyloidosis. It would therefore be interesting to utilize 11C-PIB PET in diagnosis of disease onset in mutation carriers with follow-up.
Limitations
The study was performed on a small number of patients and controls. In addition, EMB was only performed on two patients, which, however, were positive. A full compartmental analysis of PIB-PET data was not performed, and might have contributed additional insights into binding of PIB to myocardial amyloid. In brain studies of Alzheimer’s patients, kinetics were best described using models of reversible binding but did not add to the clinical interpretation.
Due to the logistical reasons and high prevalence of pacemaker carriers, cardiac MRI is not performed routinely at our center and none of the patients underwent such investigation in conjunction with the study.
Summary
The present study evaluated two different aspects of molecular imaging in a well-characterized cohort of patients with a certain diagnosis of ATTR V30M. All patients had myocardial PIB uptake on PET scan, regardless of fibril type or DPD uptake, but with significantly higher PIB retention in patients with full-length fibril type, generally associated with less cardiac involvement. Myocardial DPD uptake, on the other hand, was only elevated in patients with the fragmented fibril type.
Since 11C-PIB RI appears influenced by factors other than mere amyloid burden, its use for amyloid quantification in clinical practice might be limited. 11C-PIB PET and perfusion studies may be useful to further study amyloid depositions in vivo and their impact on tissue metabolism. It would be interesting to follow disease progression and also to measure the effect of medical treatment on cardiac amyloid deposition and tissue metabolism. This is attractive since it can be applied to pacemaker-treated patients, where cardiac magnetic resonance imaging is unsuitable.
New Knowledge Gained
C-PIB PET appears to be a very sensitive method for detecting cardiac ATTR amyloid deposits even in patients with primarily neurological disease11 and normal echocardiographic appearance. DPD scintigraphy appears to only detect ATTR deposits containing fragmented TTR, which previous studies have shown to cause more severe clinical heart disease.
Abbreviations
- ATTR:
-
Transthyretin amyloid protein
- DPD:
-
99mTechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid
- IVS:
-
Interventricular septum
- IVSD:
-
End-diastolic interventricular septum thickness
- LA:
-
Left atrium
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- 11C-PIB:
-
Carbon-11 labeled Pittsburgh compound B
- RI:
-
Retention index
- TTR:
-
Transthyretin
References
Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52:347-61.
Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014;35:E2403-12.
Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, et al. Transthyretin-related amyloidoses and the heart: A clinical overview. Nat Rev Cardiol. 2010;7:398-408.
Ihse E, Ybo A, Suhr O, Lindqvist P, Backman C, Westermark P. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol. 2008;216:253-61.
Arvidsson S, Pilebro B, Westermark P, Lindqvist P, Suhr OB. Amyloid cardiomyopathy in hereditary transthyretin V30M amyloidosis—impact of sex and amyloid fibril composition. PLoS One. 2015;10:e0143456.
Koike H, Misu K, Sugiura M, Iijima M, Mori K, Yamamoto M, et al. Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology. 2004;63:129-38.
Bergstrom J, Gustavsson A, Hellman U, Sletten K, Murphy CL, Weiss DT, et al. Amyloid deposits in transthyretin-derived amyloidosis: Cleaved transthyretin is associated with distinct amyloid morphology. J Pathol. 2005;206:224-32.
Ihse E, Rapezzi C, Merlini G, Benson MD, Ando Y, Suhr OB, et al. Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid. 2013;20:142-50.
Ericzon BG, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, et al. Liver transplantation for hereditary transthyretin amyloidosis: After 20 years still the best therapeutic alternative? Transplantation. 2015;99:1847-54.
Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470-8.
Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104-14.
Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjo L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med. 2013;54:213-20.
Lee SP, Lee ES, Choi H, Im HJ, Koh Y, Lee MH, et al. (11)C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging. 2015;8:50-9.
Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, et al. Imaging cardiac amyloidosis: A pilot study using (1)(8)F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:1652-62.
Park MA, Padera RF, Belanger A, Dubey S, Hwang DH, Veeranna V, et al. 18F-florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: autoradiography study. Circ Cardiovasc Imaging. 2015;8:e002954.
Kero T, Lindsjo L, Sorensen J, Lubberink M. Accurate analysis and visualization of cardiac C-PIB uptake in amyloidosis with semiautomatic software. J Nucl Cardiol 2015.
Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA, Doppler Quantification Task Force of the N, et al. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167-84.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79-108.
Delgado V, Mollema SA, Ypenburg C, Tops LF, van der Wall EE, Schalij MJ, et al. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J Am Soc Echocardiogr. 2008;21:1244-50.
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185-91.
Ericzon BG, Larsson M, Wilczek HE. Domino liver transplantation: risks and benefits. Transplant Proc. 2008;40:1130-1.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495.
Van Der Gucht A, Galat A, Rosso J, Guellich A, Garot J, Bodez D et al. [18F]-NaF PET/CT imaging in cardiac amyloidosis. J Nucl Cardiol 2015.
Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4:659-70.
Ma Y, Zhang S, Li J, Zheng DM, Guo Y, Feng J, et al. Predictive accuracy of amyloid imaging for progression from mild cognitive impairment to Alzheimer disease with different lengths of follow-up: A meta-analysis. Medicine 2014;93:e150.
Disclosure
None of the authors have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding
Supported by FAMY, FAMY Norrbotten and Amyl Foundation, Swedish Heart and Lung Foundation, Västerbotten County Council and Selander’s foundation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pilebro, B., Arvidsson, S., Lindqvist, P. et al. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J. Nucl. Cardiol. 25, 240–248 (2018). https://doi.org/10.1007/s12350-016-0638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-016-0638-5