Abstract
Background
Treatment options for metastatic renal cell carcinoma (mRCC) are rapidly expanding, and immunotherapy using checkpoint inhibitors is a first- or second-line option for most patients.
Objective
The objective of the present retrospective analysis was to explore the real-world impact of checkpoint inhibitor-based immunotherapy compared with therapy using other types of targeted therapies using a large real-world database.
Methods
RenIS, a registry of patients with mRCC was used as a data source. Outcomes were compared for cohorts treated with TKIs or mTOR inhibitors only [targeted therapy (TT) cohort] versus patients who received immunotherapy (IO) using a checkpoint inhibitor in any line of treatment (IO cohort). Data from a total of 1981 patients were extracted from the registry, including 1767 patients in the TT cohort and 214 patients in the IO cohort.
Results
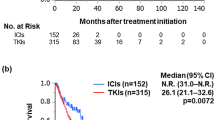
The median overall survival from the initiation of first-line treatment was 24.5 months versus not reached (p < 0.001) in the TT cohort versus the IO cohort, respectively [HR 0.23, 95% CI (0.17–0.31), p < 0.001]. The probability of 5-year survival was 24.2 versus 67.9% in the TT cohort versus the IO cohort, respectively. Immunotherapy in any line of treatment was associated with a lower risk of death. Overall survival was superior for patients receiving immunotherapy as the first or second treatment line compared with patients treated with non-immunological targeted therapy.
Conclusion
In real-world patients with mRCC, immunotherapy is associated with significant survival benefit. The present retrospective analysis shows the real-world benefit of second-line immunotherapy in patients previously treated with tyrosine–kinase inhibitors.



Similar content being viewed by others
References
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34.
Motzer RJ, Powles T, Burotto M, Escudier B, Bourlon MT, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:888–98.
Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103-15.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31.
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet (London, England). 2011;378:1931–9.
Choueiri TK, Hessel C, Halabi S, Sanford B, Michaelson MD, Hahn O, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–25.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–27.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet (London, England). 2008;372:449–56.
Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126:4156–67.
Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128:2085–97.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116-27.
Dusek L, Muzik J, Kubasek M, Koptikova J, Zaloudik J, Vyzula R. Epidemiology of malignant tumours in the czech republic [online] [internet]. Masaryk Univ. 2005 [cited 2023 May 3]. Available from: http://ww.svod.cz.
Zarbin M. Real life outcomes vs. clinical trial results. J Ophthalmic Vis Res. 2019;14:88–92.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13.
Poprach A, Bortlíček Z, Büchler T, Melichar B, Lakomý R, Vyzula R, et al. Patients with advanced and metastatic renal cell carcinoma treated with targeted therapy in the Czech Republic: twenty cancer centres, six agents, one database. Med Oncol. 2012;29:3314-20.
Kopecky J, Kubecek O, Buchler T, Melichar B, Poprach A, Zemanova M, et al. Administration of nivolumab in metastatic renal cell cancer following treatment with mtor inhibitors. In Vivo. 2021;35:2981–90.
Chakiryan NH, Jiang DD, Gillis KA, Green E, Hajiran A, Hugar L, et al. Real-world survival outcomes associated with first-line immunotherapy, targeted therapy, and combination therapy for metastatic clear cell renal cell carcinoma. JAMA Netw open. 2021;4: e2111329.
Ishihara H, Nemoto Y, Nakamura K, Tachibana H, Ikeda T, Fukuda H, et al. Comparison of outcomes between therapeutic combinations based on immune checkpoint inhibitors or tyrosine kinase inhibitor monotherapy for first-line therapy of patients with advanced renal cell carcinoma outside of clinical trials: a real-world retrospective multi-institutional study. Target Oncol. 2023;18:209–20.
Ishihara H, Nemoto Y, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Changes in real-world outcomes in patients with metastatic renal cell carcinoma from the molecular-targeted therapy era to the immune checkpoint inhibitor era. Target Oncol. 2022;17:307–19.
Santoni M, Massari F, Myint ZW, Iacovelli R, Pichler M, Basso U, et al. Global real-world outcomes of patients receiving immuno-oncology combinations for advanced renal cell carcinoma: the ARON-1 study. Target Oncol. 2023;18:559–70.
Ishihara H, Fukuda H, Takagi T, Kondo T, Tachibana H, Yoshida K, et al. Efficacy of nivolumab versus molecular-targeted therapy as second-line therapy for metastatic renal cell carcinoma: real-world data from two Japanese institutions. Int J Urol. 2021;28:99–106.
Stühler V, Herrmann L, Rausch S, Stenzl A, Bedke J. Real world data on IO-based therapy for metastatic renal cell carcinoma. J Cancer Res Clin Oncol. 2023;149:3249–58.
Gupta D, Singh A, Gupta N, Mehra N, Bahuguna P, Aggarwal V, et al. Cost-Effectiveness of the first line treatment options for metastatic renal cell carcinoma in India. JCO Glob Oncol. 2023;9: e2200246.
Shah NJ, Sura SD, Shinde R, Shi J, Singhal PK, Robert NJ, et al. Real-world treatment patterns and clinical outcomes for metastatic renal cell carcinoma in the current treatment era. Eur Urol Open Sci. 2023;49:110–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Supported by the Ministry of Health of the Czech Republic, Grant NU21-03-00539.
Conflict of interest
Alexandr Poprach has received research support from: Roche, Bristol Myers Squibb, Merck KGaA, MSD, and Novartis; consulting fees from Bristol Myers Squibb, Astellas, Janssen, and Sanofi/Aventis; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Ipsen, Bristol Myers Squibb, Roche, Novartis, Pfizer, MSD, and Pfizer. Igor Kiss has received research support from Roche, Bristol Myers Squibb, Merck KGaA, MSD, and Servier; consulting fees from Bristol Myers Squibb, Astellas, Janssen, and Sanofi/Aventis; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Ipsen, Bristol-Myers Squibb, Roche, Novartis, Pfizer, MSD, and Pfizer. Ondrej Fiala has received payment or honoraria for lectures, presentations, speakers’ bureaus, or educational events from Roche, Janssen, GSK, MSD, Pierre Fabre, BMS and Pfizer. Jindřich Kopecký has received consulting fees from Bristol Myers Squibb, Novartis, Pfizer, Merck, MSD, Ipsen; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Ipsen, Bristol Myers Squibb, MSD, Merck, Novartis and Pfizer. Igor Richter has received consulting fees from Bristol Myer Squibb, Pfizer, and Ipsen; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Ipsen, Bristol-Myers Squibb, Janssen, Merck KGaA, and Bayer. Bohuslav Melichar has received consulting fees from Roche, Pfizer, BMS, Novartis, MSD, Merck Serono, Servier, AstraZeneca, Amgen, E. Lilly. Hana Studentova has received research support: Roche, and Novartis; consulting fees from Bristol Myer Squibb, Astellas, Janssen, and MSD; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Ipsen, Bristol Myers Squibb, Janssen, and MSD. Radek Lakomy has received consulting fees from Bristol Myers Squibb, MSD, Novartis; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol-Myers Squibb, MSD, Novartis, Sanofi/Aventis and Medison. Milos Holanek has received research support from Gilead, AstraZeneca, Novartis, Roche; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Roche, Novartis, Gilead, Pfizer, AstraZeneca, Amgen. Anezka Zemankova has received consulting fees from Bristol Myer Squibb, Ipsen, Merck; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Bristol Myers Squibb, Ipsen, Merck, Pfizer and Servier. Tomas Buchler has received research support: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Merck KGaA, MSD, and Novartis; consulting fees from Bristol Myers Squibb, Astellas, Janssen, and Sanofi/Aventis; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Ipsen, Bristol Myers Squibb, AstraZeneca, Roche, Servier, Accord, MSD, and Pfizer. All unrelated to the present paper. All of the above are unrelated to the present paper. Marek Svoboda, Michal Stanik, Tamara Barusova, Lenka Pospisilova, and Aneta Rozsypalova declare no conflicts of interest.
Ethics approval
The RENIS registry has been approved by the Ethical Committee of the Brno University Hospital.
Availability of data
The datasets generated during and analysed during the current study are not publicly available due to GDPR requirements but are available in fully anonymous version from the corresponding author on reasonable request.
Author contributions
Conceptualization: AP, TB, IK. Methodology: AP, TB, TB, LP. Data Acquisition: AP, IK, MS, OF, JK, IR, BM, HS, RL, MH, AR, AZ. Statistical analysis: TB, LP. Writing—Original Draft: AP, TB. Writing—Review and Editing: all authors. Supervision: IK, TB, MS.
Consent to participate
Informed consent was acquired from participants prior to their inclusion in the database in accordance with the Ethical Committee approval and national regulations.
Code availability
Not applicable.
Consent for publication
All authors agree with the publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poprach, A., Kiss, I., Stanik, M. et al. Impact of Immunotherapy on Real-World Survival Outcomes in Metastatic Renal Cell Carcinoma. Targ Oncol 18, 893–903 (2023). https://doi.org/10.1007/s11523-023-01013-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01013-0