Abstract
Background
Clinical trials have demonstrated the superior efficacy of immune checkpoint inhibitor (ICI)-based combination therapy over sunitinib, a multi-target tyrosine kinase inhibitor (TKI), in patients with advanced renal cell carcinoma. However, such benefits have not been elucidated in populations outside of clinical trials.
Methods
We retrospectively evaluated data from 467 patients with advanced renal cell carcinoma who received ICI-based combination therapy or TKIs, as first-line therapy. Clinical outcome was compared between ICI-based combination therapy and TKIs in each population divided according to trial eligibility.
Results
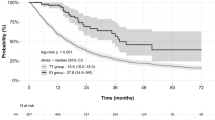
Among 152 patients treated with ICI-based combination therapy and 315 patients treated with TKIs, 76 (50.0%) and 156 (49.5%) were trial ineligible, respectively. Overall survival (p = 0.0072) and objective response rate (p < 0.0001) were significantly higher in ICI-based combination therapy than in TKIs, but progression-free survival was comparable (p = 0.681). In the trial-eligible population, overall survival was longer (p = 0.0906) and the objective response rate was significantly higher (p = 0.0124) in ICI-based combination therapy than in TKIs. In the trial-ineligible population, overall survival (p = 0.0208) and objective response rate (p = 0.0006) were significantly higher with ICI-based combination therapy than with TKIs. A multivariate analysis also showed that ICI-based combination therapy was independently associated with prolonged overall survival (hazard ratio, 0.47; p = 0.0016). Regardless of trial eligibility, progression-free survival did not differ between ICI-based combination therapy and TKIs (trial eligible: p = 0.287; trial ineligible: p = 0.0708).
Conclusions
The present study, using real-world data, provides evidence indicating the therapeutic benefit of ICI-based combination therapy over TKIs for advanced renal cell carcinoma was more statistically significant in the trial-ineligible population than in the trial-eligible population.




Similar content being viewed by others
References
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–41.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410.
Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. Kidney cancer, Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:71–90.
Rathmell WK, Rumble RB, Van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–95.
Stühler V, Herrmann L, Rausch S, Stenzl A, Bedke J. Real world data on IO-based therapy for metastatic renal cell carcinoma. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04173-0.
Ishihara H, Nemoto Y, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Changes in real-world outcomes in patients with metastatic renal cell carcinoma from the molecular-targeted therapy era to the immune checkpoint inhibitor era. Target Oncol. 2022;17:307–19.
Heng DY, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25:149–54.
Mol L, Koopman M, van Gils CW, Ottevanger PB, Punt CJ. Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in The Netherlands. Acta Oncol. 2013;52:950–5.
Knauf W, Aldaoud A, Hutzschenreuter U, Klausmann M, Dille S, Wetzel N, et al. Survival of non-transplant patients with multiple myeloma in routine care differs from that in clinical trials: data from the prospective German Tumour Registry Lymphatic Neoplasms. Ann Hematol. 2018;97:2437–45.
Marschner N, Staehler M, Müller L, Nusch A, Harde J, Koska M, et al. Survival of patients with advanced or metastatic renal cell carcinoma in routine practice differs from that in clinical trials: analyses from the German Clinical RCC Registry. Clin Genitourin Cancer. 2017;15:e209–15.
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–63.
Ishihara H, Tachibana H, Fukuda H, Yoshida K, Kobayashi H, Takagi T, et al. Prognostic impact of trial-eligibility criteria in patients with metastatic renal cell carcinoma. Urol Int. 2022;106(4):368–75.
Nemoto Y, Ishihara H, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Efficacy and safety of immunotherapy-based combinations as first-line therapy for metastatic renal cell carcinoma in patients who do not meet trial eligibility criteria. Target Oncol. 2022;17:475–82.
Fukuokaya W, Yanagisawa T, Hashimoto M, Yamamoto S, Koike Y, Imai Y, et al. Effectiveness of pembrolizumab in trial-ineligible patients with metastatic urothelial carcinoma. Cancer Immunol Immunother. 2022. https://doi.org/10.1007/s00262-022-03291-5.
Gan CL, Stukalin I, Meyers DE, Dudani S, Grosjean HAI, Dolter S, et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115–25.
Lee JL, Kim MK, Park I, Ahn JH, Lee DH, Ryoo HM, et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol. 2015;26:2300–5.
Bracarda S, Iacovelli R, Boni L, Rizzo M, Derosa L, Rossi M, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol. 2015;26:2107–13.
Kondo T, Takagi T, Kobayashi H, Iizuka J, Nozaki T, Hashimoto Y, et al. Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma—comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off. Jpn J Clin Oncol. 2014;44:270–7.
Kennoki T, Kondo T, Kimata N, Murakami J, Ishimori I, Nakazawa H, et al. Clinical results and pharmacokinetics of sorafenib in chronic hemodialysis patients with metastatic renal cell carcinoma in a single center. Jpn J Clin Oncol. 2011;41:647–55.
Ishihara H, Fukuda H, Tachibana H, Yoshida K, Kobayashi H, Takagi T, et al. Outcome of advanced renal cell carcinoma arising in end-stage renal disease: comparison with sporadic renal cell carcinoma. Clin Exp Nephrol. 2021;25:674–82.
Frampton JE. Pazopanib: a review in advanced renal cell carcinoma. Target Oncol. 2017;12:543–54.
Tachibana H, Kondo T, Ishihara H, Fukuda H, Yoshida K, Takagi T, et al. Modest efficacy of nivolumab plus ipilimumab in patients with papillary renal cell carcinoma. Jpn J Clin Oncol. 2021;51:646–53.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Rizzo M, Cartenì G, Pappagallo G. We need both randomized trials and real-world data: the example of everolimus as second-line therapy for mRCC. Future Oncol. 2014;10:1893–6.
Parikh RB, Min EJ, Wileyto EP, Riaz F, Gross CP, Cohen RB, et al. Uptake and survival outcomes following immune checkpoint inhibitor therapy among trial-ineligible patients with advanced solid cancers. JAMA Oncol. 2021;7:1843–50.
Cella D, Grünwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20:297–310.
Motzer R, Porta C, Alekseev B, Rha SY, Choueiri TK, Mendez-Vidal MJ, et al. Health-related quality-of-life outcomes in patients with advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab or everolimus versus sunitinib (CLEAR): a randomised, phase 3 study. Lancet Oncol. 2022;23:768–80.
Rzeniewicz K, Larkin J, Menzies AM, Turajlic S. Immunotherapy use outside clinical trial populations: never say never? Ann Oncol. 2021;32:866–80.
Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904–11.
Kawashima A, Takayama H, Arai Y, Tanigawa G, Nin M, Kajikawa J, et al. One-month relative dose intensity of not less than 50% predicts favourable progression-free survival in sorafenib therapy for advanced renal cell carcinoma in Japanese patients. Eur J Cancer. 2011;47:1521–6.
Iwamoto K, Ishihara H, Takagi T, Kondo T, Yoshida K, Iizuka J, et al. Evaluation of relative dose intensity during the early phase of first-line sunitinib treatment using a 2-week-on/1-week-off regimen for metastatic renal cell carcinoma. Med Oncol. 2018;35:78.
Ishihara H, Takagi T, Kondo T, Iwamoto K, Tachibana H, Yoshida K, et al. Decreased relative dose intensity during the early phase of treatment impacts the therapeutic efficacy of sunitinib in metastatic renal cell carcinoma. Jpn J Clin Oncol. 2018;48:667–72.
Ishihara H, Takagi T, Kondo T, Homma C, Tachibana H, Fukuda H, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. 2019;37(355):e21–9.
Ikeda T, Ishihara H, Nemoto Y, Tachibana H, Fukuda H, Yoshida K, et al. Prognostic impact of immune-related adverse events in metastatic renal cell carcinoma treated with nivolumab plus ipilimumab. Urol Oncol. 2021;39(735):e9-16.
Motzer RJ, Escudier B, McDermott DF, Arén Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8: e000891.
Albiges L, Guegan J, Le Formal A, Verkarre V, Rioux-Leclercq N, Sibony M, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20:3411–21.
Recondo G, Che J, Jänne PA, Awad MM. Targeting MET dysregulation in cancer. Cancer Discov. 2020;10:922–34.
Pal SK, Tangen C, Thompson IM Jr, Balzer-Haas N, George DJ, Heng DYC, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397:695–703.
Lee CH, Voss MH, Carlo MI, Chen YB, Zucker M, Knezevic A, et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol. 2022;40:2333–41.
Hayami N, Ubara Y, Okaneya T, Fujii T, Nagashima Y, Ohashi K. Outcome of renal cell carcinoma in patients on dialysis compared to non-dialysis patients. Semin Dial. 2020;33(4):316–21.
Vitale MG, Baldessari C, Milella M, Buti S, Militello AM, Di Girolamo S, et al. Immunotherapy in dialysis-dependent cancer patients: our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer. 2019;17:e903–8.
Tachibana H, Kondo T, Ishihara H, Takagi T, Tanabe K. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma and end-stage renal disease at 2 centers. Clin Genitourin Cancer. 2019;17:e772–8.
Tomita Y, Kondo T, Kimura G, Inoue T, Wakumoto Y, Yao M, et al. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: analysis of Japanese patients in CheckMate 214 with extended follow-up. Jpn J Clin Oncol. 2020;50:12–9.
Uemura M, Tomita Y, Miyake H, Hatakeyama S, Kanayama HO, Numakura K, et al. Avelumab plus axitinib vs sunitinib for advanced renal cell carcinoma: Japanese subgroup analysis from JAVELIN Renal 101. Cancer Sci. 2020;111:907–23.
Tamada S, Kondoh C, Matsubara N, Mizuno R, Kimura G, Anai S, et al. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. Int J Clin Oncol. 2022;27:154–64.
Acknowledgements
The authors thank Ms. Nobuko Hata (Department of Urology, Tokyo Women’s Medical University) for her secretarial efforts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflicts of interest/competing interests
Toshio Takagi received honoraria from BristolMyers Squibb and Ono Pharmaceutical. Tsunenori Kondo received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, and Ono Pharmaceutical. Hiroki Ishihara, Yuki Nemoto, Kazutaka Nakamura, Hidekazu Tachibana, Takashi Ikeda, Hironori Fukuda, Kazuhiko Yoshida, Hirohito Kobayashi, Junpei Iizuka, Hiroaki Shimmura, and Yasunobu Hashimoto have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study protocol was approved by the Institutional Ethics Review Board of each institution (Tokyo Women’s Medical University, Tokyo Women’s Medical University Adachi Medical Center, Saiseikai Kawaguchi General Hospital, Saiseikai Kazo Hospital, and Jyoban Hospital; ID: 2020-0009). The present study was performed according to the guidelines of the 1964 Declaration of Helsinki and its later amendments. Owing to the retrospective observational nature of this study, the need for informed consent was waived.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to this study’s conception and design, data collection, and analysis. The first draft of the manuscript was written by HI and all authors commented on the previous drafts of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ishihara, H., Nemoto, Y., Nakamura, K. et al. Comparison of Outcomes Between Therapeutic Combinations Based on Immune Checkpoint Inhibitors or Tyrosine Kinase Inhibitor Monotherapy for First-Line Therapy of Patients with Advanced Renal Cell Carcinoma Outside of Clinical Trials: A Real-World Retrospective Multi-Institutional Study. Targ Oncol 18, 209–220 (2023). https://doi.org/10.1007/s11523-023-00956-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00956-8