Abstract
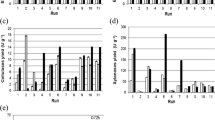
Earthworms are useful soil-decomposing animals that possess various saccharification enzymes such as cellulases and amylases. Earthworms have also been traditionally used as antipyretic agents and medicines for preventing thrombotic diseases such as brain infarction. We previously developed a novel earthworm dietary supplement with fibrinolytic, cellulase, and amylase activities using high-pressure technology. However, the optimal temperature and pH required for amylase activity in bioindustry have not yet been investigated. In the present study, we purified and characterized two α-amylases of Eisenia fetida Waki, EfAMY1 and EfAMY2, which were monomeric enzymes of 63.8 kDa and 64.0 kDa, with specific activities of 69.2 and 40.4 units/mg, respectively. The optimal pH was 5.5 for both enzymes, and the optimal temperatures were 45 °C and 35 °C for EfAMY1 and EfAMY2, respectively; however, the enzymes were stable over a wide pH range (5–10) and at high temperature (up to 40 °C). These amylases showed higher specific activity and cold tolerance than those previously reported. These data should help to promote the development of E. fetida AMYs as functional dietary supplements and in biomass utilization.





Similar content being viewed by others
References
Akazawa S, Ikarashi Y, Yarimizu J, Yokoyama K, Kobayashi T, Nakazawa H, Ogasawara W, Morikawa Y (2016) Characterization of two endoglucanases for the classification of the earthworm, Eisenia fetida waki. Biosci Biotechnol Biochem 80:55–66
Akazawa S, Tokuyama H, Sato S, Watanabe T, Shida Y, Ogasawara W (2018) High-pressure tolerance of earthworm fibrinolytic and digestive enzymes. J Biosci Bioeng 125:155–159
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Elarbi MB, Khemiri H, Jridi T, Hamida JB (2009) Purification and characterization of α-amylase from safflower (Carthamus tinctorius L.) germinating seeds. C R Biol 332:426–432
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu J, Zhang Z, Dang H, Lu J, Cui Z (2011a) Isolation and characterization of a cold-active amylase from marine Wangia sp. C52. Afr J Microbiol Res 5:1156–1162
Liu J, Zhang Z, Zhu H, Dang H, Lu J, Cui Z (2011b) Isolation and characterization of α-amylase from marine Pseudomonas sp. K6-28-040. Afr J Biotechnol 10:2733–2740
Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikeda R, Seiki M, Maruyama M (1991) A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn J Physiol 41:461–472
Russell NJ (2000) Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4:83–90
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Tracey MV (1951) Cellulase and chitinase of earthworms. Nature 167:776–777
Ueda M, Asano T, Nakazawa M, Miyatake K, Inouye K (2008) Purification and characterization of novel raw-starch-digesting and cold-adapted alpha-amylases from Eisenia foetida. Comp Biochem Physiol B Biochem Mol Biol 150:125–130
Zhang JW, Zeng RY (2008) Purification and characterization of a cold-adapted α-amylase produced by Nocardiopsis sp. 7326 isolated from Prydz Bay, Antarctic. Mar Biotechnol 10:75–82
Acknowledgments
We would like to thank Waki Pharmaceutical Co., Ltd., for supporting our research.
Funding
This work was supported in part by Grant-in-Aid from the Japan Science and Technology Agency (JST) on Adaptable and Seamless Technology Transfer Program (A-STEP) through target-driven R&D [grant number AS251Z02531L].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Chris Lowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akazawa, Si., Ikarashi, Y., Yokoyama, K. et al. Characterization of earthworm α-amylases for dietary supplement development and biomass utilization. Environ Sci Pollut Res 27, 33458–33463 (2020). https://doi.org/10.1007/s11356-019-05133-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05133-x