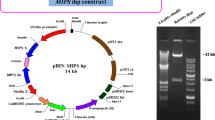
Abstract
Phytic acid (PA) is poorly digested by humans and monogastric animals and negatively affects human/animal nutrition and the environment. Rice mutants with reduced PA content have been developed but are often associated with reduced seed weight and viability, lacking breeding value. In the present study, a new approach was explored to reduce seed PA while attaining competitive yield. The OsMRP5 gene, of which mutations are known to reduce seed PA as well as seed yield and viability, was down-regulated specifically in rice seeds by using an artificial microRNA driven by the rice seed specific promoter Ole18. Seed PA contents were reduced by 35.8–71.9 % in brown rice grains of transgenic plants compared to their respective null plants (non-transgenic plants derived from the same event). No consistent significant differences of plant height or number of tillers per plant were observed, but significantly lower seed weights (up to 17.8 % reduction) were detected in all transgenic lines compared to null plants, accompanied by reductions of seed germination and seedling emergence. It was observed that the silencing of the OsMRP5 gene increased the inorganic P (Pi) levels (up to 7.5 times) in amounts more than the reduction of PA-P in brown rice. This indicates a reduction in P content in other cellular compounds, such as lipids and nucleic acids, which may affect overall seed development. Put together, the present study demonstrated that seed specific silencing of OsMRP5 could significantly reduce the PA content and increase Pi levels in seeds; however, it also significantly lowers seed weight in rice. Discussions were made regarding future directions towards producing agronomically competitive and nutritionally valuable low PA rice.




Similar content being viewed by others
References
Ali N, Paul S, Gayen D, Sarkar SN, Datta K, Datta SK (2013a) Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene (IPK1). PLoS ONE 8(7):e68161. doi:10.1371/journal.pone.0068161
Ali N, Paul S, Gayen D, Sarkar SN, Datta SK, Datta K (2013b) RNAi mediated down regulation of myo-inositol-3-phosphate synthase to generate low phytate rice. Rice 6:12. doi:10.1186/1939-8433-6-12
Chen PS, Toribara TY, Warner H (1956) Microdetermination of phosphorous. Anal Chem 28:1756–1758
Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrob-acterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16:664–670
Feng XG, Yoshida KT (2004) Molecular approaches for producing low-phytic-acid grains in rice. Plant Biotechnol 21:183–189
Hansen TH, Laursen KH, Persson DP, Pedas P, Husted S, Schjoerring JK (2009) Micro-scaled high-throughput digestion of plant tissue samples for multi-elemental analysis. Plant Methods 5:12
Hiei Y, Komari T (2008) Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 3:824–834
Hitz WD, Carlson TJ, Kerr PS, Sebastian SA (2002) Biochemical and molecular characterization of a mutation that confers a decreased raffinosaccharide and phytic acid phenotype on soybean seeds. Plant Physiol 128:650–660
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Josefsen L, Bohn L, Sorensen MB, Rasmussen SK (2007) Characterization of a multifuncti-onal inositol phosphate kinase from rice and barley belonging to the ATP-grasp superfamily. Gene 397:114–125
Kim SI, Andaya CB, Goyal SS, Tai TH (2008a) The rice OsLpa1 gene encodes a novel protein involved in phytic acid metabolism. Theor Appl Genet 117:769–779
Kim SI, Andaya CB, Newman JW, Goyal SS, Tai TH (2008b) Isolation and characterization of a low phytic acid rice mutant reveals a mutation in the rice orthologue of maize MIK. Theor Appl Genet 117:1291–1301
Kuwano M, Ohyama A, Tanaka Y, Mimura T, Takaiwa F, Yoshida KT (2006) Molecular breeding for transgenic rice with low-phytic-acid phenotype through manipulating myo-inositol 3-phosphate synthase gene. Mol Breed 18:263–272
Kuwano M, Mimura T, Takaiwa F, Yoshida KT (2009) Generation of stable ‘low phytic acid’ transgenic rice through antisense repression of the 1D-myo-inositol 3-phosphate synthase gene (RINO1) using the 18-kDa oleosin promoter. Plant Biotechnol J 7:96–105
Larson SR, Rutger JN, Young KA, Raboy V (2000) Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Sci 40:1397–1405
Li CY, Park DS, Won SR, Hong SK, Ham JK, Choi JK, Rhee HI (2008) Food chemical properties of low-phytate rice cultivar, Sang-gol. J Cereal Sci 47:262–265
Liu QL, Xu XH, Ren XL, Fu HW, Wu DX, Shu QY (2007) Generation and characterization of low phytic acid germplasm in rice (Oryza sativa L.). Theor Appl Genet 114:803–814
Loewus F, Murthy PPN (2000) Myo-Inositol metabolism in plants. Plant Sci 150:1–19
Lott JNA, Ockenden I, Raboy V, Batten GD (2000) Phytic acid and phosphorus in crops seeds and fruits: a global estimate. Seed Sci Res 10:11–33
Maroof MAS, Glover NM, Biyashev RM, Buss GR, Grabau EA (2009) Genetic basis of the low-phytate trait in the soybean line CX1834. Crop Sci 49:69–76
Nagy R, Grob H, Weder B, Green P, Klein M, Frelet-Barrand A, Schjoerring JK, Brearley C, Martinoia E (2009) The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage. J Biol Chem 284:33614–33622
Nunes AC, Vianna GR, Cuneo F, Amaya-Farfan J, de Capdeville G, Rech EL, Aragao FJ (2006) RNAi-mediated silencing of the myo-inositol-1-phosphate synthase gene (GmMIPS1) in transgenic soybean inhibited seed development and reduced phytate content. Planta 224:125–132
O’Dell BL, de Boland AR, Koirtyohann SR (1972) Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J Agric Food Chem 20:718–721
Panzeri D, Cassani E, Doria E, Tagliabue G, Forti L, Campion B, Bollini R, Brearley CA, Pilu R, Nielsen E, Sparvoli F (2011) A defective ABC transporter of the MRP family, responsible for the bean lpa1 mutation, affects the regulation of the phytic acid pathway, reduces seed myo-inositol and alters ABA sensitivity. New Phytol 191:70–83
Preiss J (1997) Modulations of starch synthesis. In: Foyer CH, Quick QP (eds) A molecular approach to primary metabolism in higher plants. Taylor and Francis, London, pp 81–104
Qu LQ, Takaiwa K (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J 2:113–125
Raboy V (2009) Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci 177:281–296
Raboy V, Young KA, Dorsch JA, Cook A (2001) Genetics and breeding of seed phosphorus and phytic acid. J Plant Physiol 158:489–497
Ren XL, Liu QL, Fu HW, Wu DX, Shu QY (2007) Density alteration of nutrient elements in rice grains of a low phytate mutant. Food Chem 102:1400–1406
Rutger JN, Raboy V, Moldenhauer KAK, Bryant RJ, Lee FN, Gibbons JW (2004) Registration of KBNT lpa1-1 low phytic acid germplasm of rice. Crop Sci 44:363
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shi JR, Wang HY, Wu YS, Hazebroek J, Meeley RB, Ertl DS (2003) The maize low-phytic acid mutant lpa2 is caused by mutation in an inositol phosphate kinase gene. Plant Physiol 131:507–515
Shi JR, Wang HY, Hazebroek J, Ertl DS, Harp T (2005) The maize low-phytic acid 3 encodes a myo-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds. Plant J 42:708–719
Shi JR, Wang HY, Schellin K, Li BL, Faller M, Stoop JM, Meeley RB, Ertl DS, Ranch JP, Glassman K (2007) Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nature Biotechnol 25:930–937
Shu QY, Cui HR, Ye GY, Wu DX, Xia YW, Gao MW, Altosaar I (2002) Agronomic and morphological characterization of agrobacterium-transformed but rice plants. Euphytica 127:345–352
Smidansky ED, Clancy M, Meyer FD, Lanning SP, Blake NK, Talbert LE, Giroux MJ (2002) Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc Natl Acad Sci USA 99:1724–1729
Smidansky ED, Martin JM, Hannah LC, Fischer AM, Giroux MJ (2003) Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216:656–664
Spear JD, Fehr WR (2007) Genetic improvement of seedling emergence of soybean lines with low phytate. Crop Sci 47:1354–1360
Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD (2005) Generation of phytate-free seeds in arabidopsis through disruption of inositol polyphosphate kinases. Proc Natl Acad Sci USA 102:12612–12617
Suzuki M, Tanaka K, Kuwano M, Yoshida KT (2007) Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): implications for the phytic acid biosynthetic pathway. Gene 405:55–64
Tan YY, Fu HW, Zhao HJ, Lu S, Fu JJ, Li YF, Cui HR, Shu QY (2013) Functional molecular markers and high-resolution melting curve analysis of low phytic acid mutations for marker-assisted selection in rice. Mol Breed 31:517–528
Trimble LA, Fehr WR (2010) Genetic improvement of seedling emergence of low-phytate soybean lines. Crop Sci 50(1):67–72
Warthmann N, Chen H, Ossowski S, Weigel D, Hervé P (2008) Highly specific gene silencing by artificial miRNAs in rice. PLoS ONE 3:e1829
Wilcox JR, Premachandra GS, Young KA, Raboy V (2000) Isolation of high seed inorganic P, low-phytate soybean mutants. Crop Sci 40:1601–1605
Wu LS, Wang LD, Chen PW, Chen LJ, Tzen JT (1998) Genomic cloning of 18 kDa oleosin and detection of triacylglyc-erols and oleosin isoforms in maturing rice and postgerminative seedlings. J Biochem 123:386–391
Xu XH, Zhao HJ, Liu QL, Fank T, Engel KH, An G, Shu QY (2009) Mutations of the multi-drug resistance-associated protein ABC transporter gene 5 result in reduction of phytic acid in rice seeds. Theor Appl Genet 119:75–83
Yadav P, Mukherjee SK (2012) Artificial microRNA and its applications. In: Mallick B, Ghosh Z (eds) Regulatory RNAs. Springer, New York, pp 505–521
Yuan FJ, Zhao HJ, Ren XL, Zhu SL, Shu QY (2007) Generation and characterization of two novel low phytate mutations in soybean (Glycine max L. Merr.). Theor Appl Genet 115:945–957
Yuan FJ, Zhu DH, Tan YY, Dong DK, Fu XJ, Zhu SL, Li BQ, Shu QY (2012) Identification and characterization of the soybean IPK1 ortholog of a low phytic acid mutant reveals an exon-excluding splice-site mutation. Theor Appl Genet 125:1413–1423
Zhao HJ, Liu QL FUHW, Xu HX, Wu DX, Shu QY (2008) Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice. Field Crops Res 108:206–211
Acknowledgments
The research was financially supported by the Natural Science Foundation of China through research grant No. 31071481, and in part by the Sino-Swiss Joint Research Project (2009 DFA32040 to QS and IZLCZ3 123946I to YP) and by Wuxi Science and Technology.Department (Grant #CYES1002), Zhejiang Provincial Innovation Team of Nuclear Agricultural Science and Technology (2010R50033). We are grateful to Dr. Yuwei Shen of DNA LandMarks for his critical comments on and improvement of the manuscript. Technical assistance of Ms. Lijuan Mao for measurement of phytic acid content is highly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, WX., Zhao, HJ., Pang, WQ. et al. Seed-specific silencing of OsMRP5 reduces seed phytic acid and weight in rice. Transgenic Res 23, 585–599 (2014). https://doi.org/10.1007/s11248-014-9792-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-014-9792-1