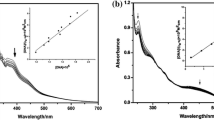
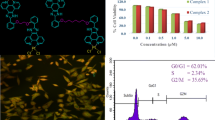
Abstract
Four unsymmetrical oxovanadium phenanthroimidazole complexes, [VO(hntdtsc)(NPIP)] (1), [VO(hntdtsc)(CPIP)] (2), [VO(hntdtsc)(MEPIP)] (3) and [VO(hntdtsc)(HPIP)] (4) (hntdtsc = 2-hydroxy-1-naphthaldehyde thiosemicarbazone, NPIP = 2-(4-nitrophenyl)-imidazo[4,5-f]1,10-phenanthroline, CPIP = 2-(4-chlorphenyl)-imidazo[4,5-f]1,10-phenanthroline), MEPIP = 2-(4-methylphenyl)-imidazo[4,5-f]1,10-phenanthroline), HPIP = 2-(4-hydroxylphenyl)-imidazo[4,5-f] 1,10-phenanthroline), have been synthesized and characterized. Their DNA binding and antitumor activities were determined by biochemical methods. All four oxovanadium complexes can bind with CT-DNA by an intercalation model and can also cleave supercoiled plasmid DNA in the presence of H2O2. The antitumor properties and mechanism of the complexes have been analyzed by MTT assay, cell cycle analysis, apoptosis assay and Western blot analysis. The results showed that the free ligands and their corresponding complexes all possess antiproliferative activities with very low IC50 values against Hela, BIU-87 and SPC-A-1 cell lines. Complex 1, which has a strongly electron-withdrawing nitro group, exhibited the best antiproliferative activities. Complex 1 caused G0/G1 phase arrest of the cell cycle and induced apoptosis in Hela cells. Additionally, complex 1 attenuated the phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2).This indicates that inhibition of the ERK1/2 signaling pathway may contribute to the antitumor effects of these complexes.










Similar content being viewed by others
References
Leon IE, Cadavid-Vargas JF, Di Virgilio AL, Etcheverry S (2017) Vanadium, ruthenium and copper compounds: a new class of nonplatinum metallodrugs with anticancer activity. Curr Med Chem 24(2):112–148
Sanna D, Ugone V, Fadda A, Micera G, Garribba E (2016) Behavior of the potential antitumor V(IV)O complexes formed by flavonoid ligands. 3. Antioxidant properties and radical production capability. J Inorg Biochem 161:18–26
Andrezalova L, Gbelcova H, Durackova Z (2013) DNA damage induction and antiproliferative activity of vanadium(V) oxido monoperoxido complex containing two bidentate heteroligands. J Trace Elem Med Biol 27:21–26
Wang G, Wang J, Fu Y, Bai L, He M, Li B, Fu Q (2013) Systemic treatment with vanadium absorbed by Coprinus comatus promotes femoral fracture healing in streptozotocin-diabetic rats. Biol Trace Elem Res 151:424–433
Reddy GNR, Kondaiah S, Setty KN, Rao RM, Ramulu JS (2012) Synthesis, structural characterization and anti-bacterial activity of some novel Schiff base ligands and their vanadium(IV) metal complexes. Orient J Chem 28(4):1673–1683
Park S-J, Youn C-K, Hyun JW, You HJ (2013) The anti-obesity effect of natural vanadium-containing Jeju ground water. Biol Trace Elem Res 151(2):294–300
Holko P, Ligeza J, Kisielewska J, Kordowiak AM, Klein A (2008) The effect of vanadyl sulphate (VOSO4) on autocrine growth of human epithelial cancer cell lines. Pol J Pathol 59:3–8
Klein A, Holko P, Ligeza J, Kordowiak AM (2008) Sodium orthovanadate affects growth of some human epithelial cancer cells (A549, HTB44, DU145). Folia Biol (Krakow) 56:115–121
Molinuevo MS, Cortizo AM, Etcheverry SB (2008) Vanadium(IV) complexes inhibit adhesion, migration and colony formation of UMR106 osteosarcoma cells. Cancer Chemother Pharmacol 61:767–773
Rodríguez-Mercado JJ, Mateos-Nava RA, Altamirano-Lozano MA (2011) DNA damage induction in human cells exposed to vanadium oxides in vitro. Toxicol In Vitro 25:1996–2002
Soares SS, Henao F, Aureliano M, Gutiérrez-Merino C (2008) Vana-date induces necrotic death in neonatal rat cardiomyocytes through mitochondrial membrane depolarization. Chem Res Toxicol 21:607–618
Zhao Y, Ye L, Liu H, Xia Q, Zhang Y, Yang X, Wang K (2010) Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J Inorg Biochem 104:371–378
Pessoa JC, Etcheverry SB, Gambino D (2015) Vanadium compounds in medicine. Coord Chem Rev 301:24–48
Ying P, Zeng P, Jiazheng L, Chen H, Liao X, Yang N (2015) New oxidovanadium complexes incorporating thiosemicarbazones and 1, 10-phenanthroline derivatives as DNA cleavage, potential anticancer agents, and hydroxyl radical scavenger. Chem Biol Drug Des 86:926–937
Hwang JH, Larson RK, Abu-Omar MM (2003) Kinetics and mechanistic studies of anticarcinogenic bisperoxovanadium(V) compounds: ligand substitution reactions at physiological pH and relevance to DNA interactions. Inorg Chem 42(24):7967–7977
Wu H-L, Li K, Sun T, Kou F, Jia F, Yuan J-K, Liu B, Qi B-L (2011) Synthesis, structure, and DNA-binding properties of manganese(II) and zinc(II) complexes with tris(N-methylbenzimidazol-2-ylmethyl)amine ligand. Transition Met Chem 36(1):21–28
Ji LN, Zhou XH, Liu JG (2001) DNA-binding activities and potential antitumor properties of some ruthenium complexes. Coord Chem Rev 216–217(1):513–516
Leelavathy L, Anbu S, Kandaswamy M et al (2009) Antibacterium properties of some porphyrin derivatives. Polyhedron 28(2):903–910
Fiel RJ, Munson BR (1980) Binding of meso-tetra(4-N-methylpyridyl)porphine to DNA. Nucleic Acids Res 8(12):2835–2842
Pasternack RF, Gibbs EJ, Villafranca JJ (1983) Possible mechanism of potential anticancer properties of some ruthenium complexes. Biochemistry 22(2):2406–2414
Satyanarayana S, Dabrowiak JC, Chaires JB (1992) Synthesis, characterization and DNA-binding activities of porphyrins. J Biochem 31(4):9319–9324
Uno T, Hamasaki K, Tanigawa M, Shimabayashi S (1997) Binding of meso-tetrakis(N-methylpyridinium-4-yl)porphyrin to double helical RNA and DNA–RNA Hybrids. Inorg Chem 36(8):1676–1683
Kovala-Demertzi D, Demertzis MA, Miller JR, Papadopoulou C, Dodorou C, Filousis G (2001) Platinum(II) complexes with 2-acetylpyridine thiosemicarbazone. Synthesis, crystal structure, spectral properties, antimicrobial and antitumour activity. J Inorg Biochem 86(2–3):555–563
McGhee JD (1976) Theoretical calculations of the helix-coil transition of DNA in the presence of large, cooperatively binding ligands. Biopolymers 15(7):1345–1375
Waring MJ (1965) Complex formation between ethidium bromide and nucleic acids. J Mol Biol 13(1):269–282
Barton JK, Raphael AL (1984) Photoactivated stereospecific cleavage of double-helical DNA by cobalt(III) complexes. J Am Chem Soc 106(8):2466–2468
Zhang Q-L, Liu J-G, Liu J-Z, Li H, Yang Y, Xu H, Chao H, Ji L-N (2002) Effect of intramolecular hydrogen-bond on the DNA-binding and photocleavage properties of polypyridyl cobalt(III) complexes. Inorg Chim Acta 339:34–40
Brooks HB, Sicilio F (1971) Electron spin resonance kinetic studies of the oxidation of vanadium(IV) by hydrogen peroxide. Inorg Chem 10(11):2530–2534
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) J Immunol Methods 184:39–51
Kim R (2005) Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 103(8):1551–1560
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro.ovrddot.organisms. J Mol Biol 3:208
Cohen G, Eisenberg H (1969) Viscosity and sedimentation study of sonicated DNA-proflavine complexes. Biopolymers 8(1):45–55
Benitez J, Becco L, Correia I, Leal SM, Guiset H, Pessoa JC, Lorenzo J, Tanco S, Escobar P, Moreno V, Garat B, Gambino D (2011) Vanadium polypyridyl compounds as potential antiparasitic and antitumoral agents: new achievements. J Inorg Biochem 105(2):303–312
Liang X-L, Tan L-F, Zhu W-G (2011) Study on DNA-binding and DNA-cleavage properties of Cr(III) complexes with polypyridyl ligands. DNA Cell Biol 30(1):61–67
Zhao P, Lian-Cai X, Huang J-W, Bo F, Han-Cheng Yu, Ji L-N (2008) Tricationic pyridium porphyrins appending different peripheral substituents: experimental and DFT studies on their interactions with DNA. Biophys Chem 135:102–109
Zhao P, Lian-Cai X, Huang J-W, Bo F, Han-Cheng Yu, Ji L-N (2008) Experimental and DFT studies on DNA binding and photocleavage of two cationic porphyrins: Effects of the introduction of a carboxyphenyl into pyridinium porphyrin. Spectrochim Acta Part A Mol Biomol Spectrosc 71:1216–1223
Zhao P, Lian-Cai X, Huang J-W, Bo F, Han-Cheng Yu, Ji L-N (2008) DNA binding and photocleavage properties of cationic porphyrin-anthraquinone hybrids with different lengths of links. Bioorg Chem 36:278–287
Kurita N, Kobayashi K (2000) Comput Chem 24:351–355
Jia-Zheng L, Yi-Fan D, Guo H-W (2011) Two oxovanadium complexes incorporating thiosemicarbazones: synthesis, characterization and DNA-binding studies. J Coord Chem 64(7):1229–1239
Du YF, Lu JZ, Guo HW et al (2010) Synthesis, characterization and photocleavage activities of oxovanadium complexes. Transit Metal Chem 35(7):859–864
Li C, Wu J, Wang L, Min R, Jia N, Guo J (1999) Synthesis, characterization and antitumor activity of copper(II) complex with nicotinamido-4-bis(2-chloroethyl)aminobenzaldimine. J Inorg Biochem 73(4):195–202
Liu YJ, Zeng CH, Huang HL et al (2010) DNA-binding, photocleavage and anticancer activities of some rhyseniuim complexes. J Eur Med Chem 45(12):564–571
Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen YP, Ma XY, Sui DY (2010) Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondria-dependent pathway. Eur J Pharmacol 645:14–22
Zhao Q, Bai Y, Li C et al (2017) Oleuropein protects cardiomyocyte against apoptosis via activating the reperfusion injury salvage kinase pathway in vitro. Evid Based Complement Alternat Med eCAM 2017:2109018
Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26(22):3291–3310
Montagut C, Settleman J (2009) Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett 283(2):125
Thompson N, Lyons J (2005) Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol 5(4):350
De Luca A, Maiello MR, D’Alessio A et al (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16(Suppl 2):S17
Saini KS, Loi S, De AE et al (2013) Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev 39(8):935–946
Santarpia L, Lippman SM, Elnaggar AK (2012) Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16(1):103–119
Acknowledgements
This study was supported by Natural Science Foundation of Gansu Province, China (Grant No. 145RJZA143) and Administration of traditional Chinese medicine Scientific Research Funds of Gansu Province (Grant No. GZK-2017-53). We thank Dr. Zhi-Jian Han for language editing and improvement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bai, YL., Zhang, YW., Xiao, JY. et al. Oxovanadium phenanthroimidazole derivatives: synthesis, DNA binding and antitumor activities. Transit Met Chem 43, 171–183 (2018). https://doi.org/10.1007/s11243-018-0205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0205-9