Abstract
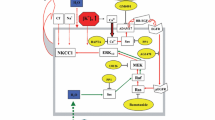
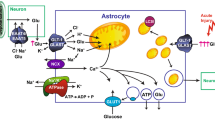
This paper describes the roles of the astrocytic Na+, K+-ATPase for K+ homeostasis in brain. After neuronal excitation it alone mediates initial cellular re-accumulation of moderately increased extracellular K+. At higher K+ concentrations it is assisted by the Na+, K+, 2Cl− transporter NKCC1, which is Na+, K+-ATPase-dependent, since it is driven by Na+, K+-ATPase-created ion gradients. Besides stimulation by high K+, NKCC1 is activated by extracellular hypertonicity. Intense excitation is followed by extracellular K+ undershoot which is decreased by furosemide, an NKCC1 inhibitor. The powerful astrocytic Na+, K+-ATPase accumulates excess extracellular K+, since it is stimulated by above-normal extracellular K+ concentrations. Subsequently K+ is released via Kir4.1 channels (with no concomitant Na+ transport) for re-uptake by the neuronal Na+, K+-ATPase which is in-sensitive to increased extracellular K+, but stimulated by intracellular Na+ increase. Operation of the astrocytic Na+, K+-ATPase depends upon Na+, K+-ATPase/ouabain-mediated signaling and K+-stimulated glycogenolysis, needed in these non-excitable cells for passive uptake of extracellular Na+, co-stimulating the intracellular Na+-sensitive site. A gradual, spatially dispersed release of astrocytically accumulated K+ will therefore not re-activate the astrocytic Na+, K+-ATPase. The extracellular K+ undershoot is probably due to extracellular hypertonicity, created by a 3:2 ratio between Na+, K+-ATPase-mediated Na+ efflux and K+ influx and subsequent NKCC1-mediated volume regulation. The astrocytic Na+, K+-ATPase is also stimulated by β1-adrenergic signaling, which further stimulates hypertonicity-activation of NKCC1. Brain ischemia leads to massive extracellular K+ increase and Ca2+ decrease. A requirement of Na+, K+-ATPase signaling for extracellular Ca2+ makes K+ uptake (and brain edema) selectively dependent upon β1-adrenergic signaling and inhibitable by its antagonists.








Similar content being viewed by others
References
Skou JC (1957) The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23:394–401
Skou JC (2004) The identification of the sodium pump. Biosci Rep 24:436–451
Henn FA, Haljamäe H, Hamberger A (1972) Glial cell function: active control of extracellular K+ concentration. Brain Res 43:437–443
Grisar T, Frere JM, Franck G (1979) Effect of K+ ions on kinetic properties of the Na+, K+-ATPase (EC 3.6.1.3) of bulk isolated glial cells, perikarya and synaptosomes from rabbit brain cortex. Brain Res 165:87–103
Hajek I, Subbarao KV, Hertz L (1996) Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 28:335–342
Hertz L, Gerkau NJ, Xu J, Durry S, Song D, Rose C, Peng L (2014) Roles of astrocytic Na+, K+-ATPase and glycogenolysis for K+ homeostasis in mammalian brain. J Neurosci Res. doi:10.1002/jnr.23499
Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelièvre L, Geering K (2000) Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem 275:1976–1986
Li B, Hertz L, Peng L (2013) Cell-specific mRNA alterations in Na+, K+-ATPase α and β isoforms and FXYD in mice treated chronically with carbamazepine, an anti-bipolar drug. Neurochem Res 38:834–841
Yoshimura K (1973) Activation of Na-K activated ATPase in rat brain by catecholamine. J Biochem 74:389–391
Godfraind T, Koch MC, Verbeke N (1974) The action of EGTA on the catecholamines stimulation of rat brain Na-K-ATPase. Biochem Pharmacol 23:3505–3511
Wu PH, Phillis JW (1979) Receptor-mediated noradrenaline stimulation of Na+–K+ ATPase in rat brain cortical homogenates. Gen Pharmacol 10:189–192
Xiong ZQ, Stringer JL (2000) Sodium pump activity, not glial spatial buffering, clears potassium after epileptiform activity induced in the dentate gyrus. J Neurophysiol 83:1443–1451
Ransom CB, Ransom BR, Sontheimer H (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522(Pt 3):427–442
D’Ambrosio R, Gordon DS, Winn HR (2002) Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J Neurophysiol 87:87–102
Somjen GG, Kager H, Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci 25:349–365
Dufour S, Dufour P, Chever O, Vallée R, Amzica F (2011) In vivo simultaneous intra- and extracellular potassium recordings using a micro-optrode. J Neurosci Methods 194:206–217
Macaulay N, Zeuthen T (2012) Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res 37:2299–2309
Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M (2012) Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5:ra26
Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, MacAulay N (2014) Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62:608–622
Walz W, Hertz L (1982) Ouabain-sensitive and ouabain-resistant net uptake of potassium into astrocytes and neurons in primary cultures. J Neurochem 39:70–77
Walz W, Hertz L (1984) Intense furosemide-sensitive potassium accumulation in astrocytes in the presence of pathologically high extracellular potassium levels. J Cereb Blood Flow Metab 4:301–304
Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ (2010) Cotransport of water by the Na+–K+–2Cl− cotransporter NKCC1 in mammalian epithelial cells. J Physiol 588:4089–4101
Zeuthen T, Macaulay N (2012) Cotransport of water by Na+–K+–2Cl− cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J Physiol 590:1139–1154
Lothman E, Lamanna J, Cordingley G, Rosenthal M, Somjen G (1975) Responses of electrical potential, potassium levels, and oxidative metabolic activity of the cerebral neocortex of cats. Brain Res 88:15–36
Hertz L, Peng L, Song D (2104) Ammonia, like K+, stimulates the Na+, K+, 2Cl− cotransporter NKCC1 and the Na+, K+-ATPase and interacts with endogenous ouabain in astrocytes. Neurochem Res [Epub ahead of print]
Pedersen SF, O’Donnell ME, Anderson SE, Cala PM (2006) Physiology and pathophysiology of Na+/H+ exchange and Na+–K+–2Cl− cotransport in the heart, brain, and blood. Am J Physiol Regul Integr Comp Physiol 291:R1–R25
Hertz L, Bender AS, Woodbury DM, White HS (1989) Potassium-stimulated calcium uptake in astrocytes and its potent inhibition by nimodipine. J Neurosci Res 22:209–215
Song D, Xu J, Hertz L, Peng L (2015) Regulatory volume increase in astrocytes exposed to hypertonic medium requires β1-adrenergic Na+/K+-ATPase stimulation and glycogenolysis. J Neurosci Res 93:130–139
Orkand RK, Nicholls JG, Kuffler SW (1996) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29:788–806
Kuffler SW, Nicholls JG (1996) The physiology of neuroglial cells. Ergeb Physiol 57:1–90
Bay V, Butt AM (2012) Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia 60:651–660
Scemes E, Spray DC (2012) Extracellular K+ and astrocyte signaling via connexin and pannexin channels. Neurochem Res 37:2310–2316
Rimmele TS, Chatton JY (2014) A novel optical intracellular imaging approach for potassium dynamics in astrocytes. PLoS One 9:e109243
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2012) The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res 37:2432–2438
Hof PR, Pascale E, Magistretti PJ (1988) K+ at concentrations reached in the extracellular space during neuronal activity promotes a Ca2+-dependent glycogen hydrolysis in mouse cerebral cortex. J Neurosci 8:1922–1928
Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38:472–485
Seip G, Schultheiss G, Kocks SL, Diener M (2001) Interaction between store-operated non-selective cation channels and the Na+–Ca2+ exchanger during secretion in the rat colon. Exp Physiol 86:461–468
Müller MS, Fox R, Schousboe A, Waagepetersen HS, Bak LK (2014) Astrocyte glycogenolysis is triggered by store-operated calcium entry and provides metabolic energy for cellular calcium homeostasis. Glia 62:526–534
Teiwes J, Toto RD (2007) Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens 20:109–117
Song D, Du T, Li B, Cai L, Gu L, Li H, Chen Y, Hertz L, Peng L (2008) Astrocytic alkalinization by therapeutically relevant lithium concentrations: implications for myo-inositol depletion. Psychopharmacology 200:187–195
Segall L, Daly SE, Blostein R (2001) Mechanistic basis for kinetic differences between the rat α1, α2, and α3 isoforms of the Na, K-ATPase. J Biol Chem 276:31535–31541
Hertz L, Xu J, Song D, Du T, Li B, Yan E, Peng L (2014) Astrocytic glycogenolysis: mechanisms and functions. Metab Brain Dis [Epub ahead of print]
Cruz NF, Ball KK, Dienel GA (2007) Functional imaging of focal brain activation in conscious rats: impact of [14C] glucose metabolite spreading and release. J Neurosci Res 85:3254–3266
Hertz L, Xu J, Song D, Yan E, Gu L, Peng L (2013) Astrocytic and neuronal accumulation of elevated extracellular K+ with a 2/3 K+/Na+ flux ratio—consequences for energy metabolism, osmolarity and higher brain function. Front Comput Neurosci 7:114
Dietzel I, Heinemann U, Hofmeier G, Lux HD (1982) Stimulus-induced changes in extracellular Na+ and Cl− concentration in relation to changes in the size of the extracellular space. Exp Brain Res 46:73–84
Dietzel I, Heinemann U, Lux HD (1989) Relations between slow extracellular potential changes, glial potassium buffering, and electrolyte and cellular volume changes during neuronal hyperactivity in cat brain. Glia 2:25–44
Thomas RC (1972) Electrogenic sodium pump in nerve and muscle cells. Physiol Rev 52:563–594
Risher WC, Andrew RD, Kirov SA (2009) Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia 57:207–221
Huang R, Somjen GG (1995) The effect of graded hypertonia on interstitial volume, tissue resistance and synaptic transmission in rat hippocampal tissue slices. Brain Res 702:181–187
Yuan H, Gao B, Duan L, Jiang S, Cao R, Xiong YF, Rao ZR (2010) Acute hyperosmotic stimulus-induced Fos expression in neurons depends on activation of astrocytes in the supraoptic nucleus of rats. J Neurosci Res 88:1364–1373
Juurlink BHJ, Hertz L (1992) Astrocytes. In: Boulton AA, Baker GB, Walz W (eds) Neuromethods in cell cultures. Humana Press, New York, pp 269–321
Hertz L, Code WE (1993) Calcium channel signalling in astrocytes. In: Paoletti R, Godfraind T, Vankoullen PM (eds) Calcium antagonists: pharmacology and clinical research. Kluwer Academic Publishers, Boston, pp 205–213
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914
Langer J, Rose CR (2009) Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol 587:5859–5877
Gibbs ME, Ng KT (1977) Counteractive effects of norepinephrine and amphetamine on ouabain-induced amnesia. Pharmacol Biochem Behav 6:533–537
Hertz L, Xu J, Song D, Du T, Yan E, Peng L (2013) Brain glycogenolysis, adrenoceptors, pyruvate carboxylase, Na+, K+-ATPase and Marie E. Gibbs’ pioneering learning studies. Front Integr Neurosci 7:20
Gibbs ME, Hutchinson DS, Summers RJ (2008) Role of beta-adrenoceptors in memory consolidation: beta3-adrenoceptors act on glucose uptake and beta2-adrenoceptors on glycogenolysis. Neuropsychopharmacology 33:2384–2397
Seidel JL, Shuttleworth CW (2011) Contribution of astrocyte glycogen stores to progression of spreading depression and related events in hippocampal slices. Neuroscience 192:295–303
Gable ME, Abdallah SL, Najjar SM, Liu L, Askari A (2014) Digitalis-induced cell signaling by the sodium pump: on the relation of Src to Na+/K+-ATPase. Biochem Biophys Res Commun 446:1151–1154
Fishman MC (1979) Endogenous digitalis-like activity in mammalian brain. Proc Natl Acad Sci USA 76:4661–4663
Leenen FHH, Harmsen E, Yu H (1994) Dietary sodium and central vs. peripheral ouabain-like activity in Dahl salt-sensitive vs. salt-resistant rats. Am J Physiol 267:H91916–H91920
Bagrov AY, Fedorova OV (2005) Cardenolide and bufadienolide ligands of the sodium pump. How they work together in NaCl sensitive hypertension. Front Biosci 10:2250–2256
Kala G, Kumarathasan R, Peng L, Leenen FH, Hertz L (2000) Stimulation of Na+, K+-ATPase activity, increase in potassium uptake, and enhanced production of ouabain-like compounds in ammonia-treated mouse astrocytes. Neurochem Int 36:203–211
Haas M, Askari A, Xie Z (2000) Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem 275:27832–27837
Zhang L, Zhang Z, Guo H, Wang Y (2008) Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol 22:615–621
Cai L, Du T, Song D, Li B, Hertz L, Peng L (2011) Astrocyte ERK phosphorylation precedes K+-induced swelling but follows hypotonicity-induced swelling. Neuropathology 31:250–264
Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79:763–854
Song H, Thompson SM, Blaustein MP (2013) Nanomolar ouabain augments Ca2+ signaling in rat hippocampal neurones and glia. J Physiol 591:1671–1689
Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM (2009) The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53:291–298
Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS (2008) Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci 28:6659–6663
Song D, Xu J, Du T, Yan E, Hertz L, Walz W, Peng L (2014) Inhibition of brain swelling after ischemia-reperfusion by β-adrenergic antagonists—correlation with increased K+ and decreased Ca2+ concentrations in extracellular fluid. Biomed Res Int 2014:873590
Hansen AJ, Nedergaard M (1998) Brain ion homeostasis in cerebral ischemia. Neurochem Pathol 9:195–209
Goyagi T, Horiguchi T, Nishikawa T, Tobe Y (2010) Post-treatment with selective β1 adrenoceptor antagonists provides neuroprotection against transient focal ischemia in rats. Brain Res 1343:213–217
Hertz L, Xu J, Chen Y, Gibbs ME, Du T, Hertz L, Xu J, Chen Y, Gibbs ME, Du T (2014) Antagonists of the vasopressin V1 receptor and of the β1-adrenoceptor inhibit cytotoxic brain edema in stroke by effects on astrocytes—but the mechanisms differ. Curr Neuropharmacol 12:308–323
Du T, Li B, Li H, Li M, Hertz L, Peng L (2010) Signaling pathways of isoproterenol-induced ERK1/2 phosphorylation in primary cultures of astrocytes are concentration-dependent. J Neurochem 115:1007–1023
Kirstein M, Eickhorn R, Langenfeld H, Kochsiek K, Antoni H (1991) Influence of beta-adrenergic stimulation on the fast sodium current in the intact rat papillary muscle. Basic Res Cardiol 86:441–448
Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM (2005) Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97:252–259
Béguin P, Crambert G, Monnet-Tschudi F, Uldry M, Horisberger JD, Garty H, Geering K (2002) FXYD7 is a brain-specific regulator of Na, K-ATPase α1-beta isozymes. EMBO J 21:3264–3273
Xu J, Song D, Bai Q, Cai L, Hertz L, Peng L (2014) Basic mechanism leading to stimulation of glycogenolysis by isoproterenol, EGF, elevated extracellular K+ concentrations, or GABA. Neurochem Res 39:661–667
Hutchinson DS, Summers RJ, Gibbs ME (2007) Beta2- and beta3-adrenoceptors activate glucose uptake in chick astrocytes by distinct mechanisms: A mechanism for memory enhancement? J Neurochem 103:997–1008
Cambron M, D’Haeseleer M, Laureys G, Clinckers R, Debruyne J, De Keyser J (2012) White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J Cereb Blood Flow Metab 32:413–424
Li J, Philip JL, Xu X, Theccanat T, Razzaque MA, Akhter SA (2014) β-Arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol 76C:73–83
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2010) Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab 30:1895–1904
Müller MS (2014) Functional impact of glycogen degradation on astrocytic signalling. Biochem Soc Trans 42:1311–1315
Öz G, Tesfaye N, Kumar A, Deelchand DK, Eberly LE, Seaquist ER (2012) Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab 32:256–263
Dienel GA, Ball KK, Cruz NF (2007) A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem 102:466–478
Müller MS, Pedersen SE, Walls AB, Waagepetersen HS, Bak LK (2015) Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63:154–162
Dienel GA, Cruz NF (2014) Contributions of glycogen to astrocytic energetics during brain activation. Metab Brain Dis [Epub ahead of print]
Fernández-Moncada I, Barros LF (2014) Non-preferential fuelling of the Na+/K+-ATPase pump. Biochem J 460:353–361
Peng L, Juurlink BH, Hertz L (1996) Pharmacological and developmental evidence that the potassium-induced stimulation of deoxyglucose uptake in astrocytes is a metabolic manifestation of increased Na+-K+-ATPase activity. Dev Neurosci 18:353–359
Hertz L, Schou M (1962) Univalent cations and the respiration of brain-cortex slices. Biochem J 85:93–104
Walz W, Mukerji S (1988) Lactate release from cultured astrocytes and neurons: a comparison. Glia 1:366–370
Ashford CA (1934) Glycolysis in brain tissue. Biochem J 28:2229–2236
Acknowledgments
The study was supported by Grants No. 31440048 from the National Natural Science Foundation of China to LP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Dr. Gerald Dienel.
Rights and permissions
About this article
Cite this article
Hertz, L., Song, D., Xu, J. et al. Role of the Astrocytic Na+, K+-ATPase in K+ Homeostasis in Brain: K+ Uptake, Signaling Pathways and Substrate Utilization. Neurochem Res 40, 2505–2516 (2015). https://doi.org/10.1007/s11064-014-1505-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1505-x