Abstract
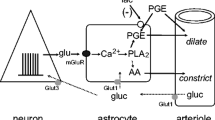
Energy homeostasis in the brain is maintained by oxidative metabolism of glucose, primarily to fulfil the energy demand associated with ionic movements in neurons and astrocytes. In this contribution we review the experimental evidence that grounds a specific role of glycogen metabolism in supporting the functional energetic needs of astrocytes during the removal of extracellular potassium. Based on theoretical considerations, we further discuss the hypothesis that the mobilization of glycogen in astrocytes serves the purpose to enhance the availability of glucose for neuronal glycolytic and oxidative metabolism at the onset of stimulation. Finally, we provide an evolutionary perspective for explaining the selection of glycogen as carbohydrate reserve in the energy-sensing machinery of cell metabolism.
Similar content being viewed by others
Abbreviations
- AK:
-
Adenylate kinase
- AMPK:
-
AMP-activated protein kinase
- CK:
-
Creatine kinase
- HK:
-
Hexokinase
- Glc-6-P:
-
Glucose 6-phosphate
- GP:
-
Glycogen phosphorylase
- GS:
-
Glycogen synthase
- KCC:
-
K+/Cl− cotransporter
- NKA:
-
Na+/K+-adenosine triphosphatase (sodium pump)
- NKCC:
-
Na+/K+/2Cl− cotransporter
- PFK:
-
Phosphofructokinase
- PKA:
-
cAMP-dependent protein kinase A
- ROS:
-
Reactive oxygen species
References
Hertz L (1965) Possible role of neuroglia: a potassium-mediated neuronal–neuroglial–neuronal impulse transmission system. Nature 206(989):1091–1094
Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27(2):219–249. doi:10.1038/sj.jcbfm.9600343
Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21(10):1133–1145. doi:10.1097/00004647-200110000-00001
Newman EA (1995) Glial cell regulation of extracellular potassium. In: Kettenmann H, Ransom BR (eds) Neuroglia. Oxford University Press, New York, pp 717–731
Ransom CB, Ransom BR, Sontheimer H (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522(Pt 3):427–442
Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R (1994) Tissue- and isoform-specific kinetic behavior of the Na, K-ATPase. J Biol Chem 269(24):16668–16676
Hertz L (1966) Neuroglial localization of potassium and sodium effects on respiration in brain. J Neurochem 13(12):1373–1387
Haljamae H, Hamberger A (1971) Potassium accumulation by bulk prepared neuronal and glial cells. J Neurochem 18(10):1903–1912
Henn FA, Haljamae H, Hamberger A (1972) Glial cell function: active control of extracellular K+ concentration. Brain Res 43(2):437–443
Hajek I, Subbarao KV, Hertz L (1996) Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 28(3):335–342
Honegger P, Pardo B (1999) Separate neuronal and glial Na+, K+-ATPase isoforms regulate glucose utilization in response to membrane depolarization and elevated extracellular potassium. J Cereb Blood Flow Metab 19(9):1051–1059. doi:10.1097/00004647-199909000-00013
Grisar T, Frere JM, Franck G (1979) Effect of K+ ions on kinetic properties of the (Na+, K+)-ATPase (EC 3.6.1.3) of bulk isolated glial cells, perikarya and synaptosomes from rabbit brain cortex. Brain Res 165(1):87–103
Mercado R, Hernandez J (1992) Regulatory role of a neurotransmitter (5-HT) on glial Na+/K(+)-ATPase in the rat brain. Neurochem Int 21(1):119–127
Walz W, Hinks EC (1986) A transmembrane sodium cycle in astrocytes. Brain Res 368(2):226–232
Walz W (2000) Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int 36(4–5):291–300. doi:S0197-0186(99)00137-0
Chever O, Djukic B, McCarthy KD, Amzica F (2010) Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J Neurosci 30(47):15769–15777. doi:10.1523/JNEUROSCI.2078-10.2010
Dufour S, Dufour P, Chever O, Vallee R, Amzica F (2011) In vivo simultaneous intra- and extracellular potassium recordings using a micro-optrode. J Neurosci Methods 194(2):206–217. doi:10.1016/j.jneumeth.2010.10.004
Somjen GG, Kager H, Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci 25(2):349–365. doi:10.1007/s10827-008-0083-9
Phelps CH (1972) Barbiturate-induced glycogen accumulation in brain. An electron microscopic study. Brain Res 39(1):225–234
Pfeiffer-Guglielmi B, Fleckenstein B, Gn Jung, Hamprecht B (2003) Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J Neurochem 85(1):73–81
DiNuzzo M, Maraviglia B, Giove F (2011) Why does the brain (not) have glycogen? BioEssays 33(5):319–326. doi:10.1002/bies.201000151
Quach TT, Rose C, Schwartz JC (1978) [3H]Glycogen hydrolysis in brain slices: responses to neurotransmitters and modulation of noradrenaline receptors. J Neurochem 30(6):1335–1341
Magistretti PJ, Morrison JH, Shoemaker WJ, Bloom FE (1983) Effect of 6-hydroxydopamine lesions on norepinephrine-induced [3H] glycogen hydrolysis in mouse cortical slices. Brain Res 261(1):159–162
Ververken D, Van Veldhoven P, Proost C, Carton H, De Wulf H (1982) On the role of calcium ions in the regulation of glycogenolysis in mouse brain cortical slices. J Neurochem 38(5):1286–1295
Pentreath VW, Kai–Kai MA (1982) Significance of the potassium signal from neurones to glial cells. Nature 295(5844):59–61
Cambray-Deakin M, Pearce B, Morrow C, Murphy S (1988) Effects of extracellular potassium on glycogen stores of astrocytes in vitro. J Neurochem 51(6):1846–1851
Subbarao KV, Stolzenburg JU, Hertz L (1995) Pharmacological characteristics of potassium-induced, glycogenolysis in astrocytes. Neurosci Lett 196(1–2):45–48
Hof PR, Pascale E, Magistretti PJ (1988) K+ at concentrations reached in the extracellular space during neuronal activity promotes a Ca2+-dependent glycogen hydrolysis in mouse cerebral cortex. J Neurosci 8(6):1922–1928
Su G, Haworth RA, Dempsey RJ, Sun D (2000) Regulation of Na(+)-K(+)-Cl(−) cotransporter in primary astrocytes by dibutyryl cAMP and high [K(+)](o). Am J Physiol Cell Physiol 279(6):C1710–1721
Vaughan H, Thornton SD, Newsholme EA (1973) The effects of calcium ions on the activities of trehalase, hexokinase, phosphofructokinase, fructose diphosphatase and pyruvate kinase from various muscles. Biochem J 132(3):527–535
Swanson RA (1992) Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol 70(Suppl):S138–S144
Dienel GA, Cruz NF (2006) Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem Int 48(6–7):586–595. doi:10.1016/j.neuint.2006.01.004
Swanson RA, Morton MM, Sagar SM, Sharp FR (1992) Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience 51(2):451–461
Dienel GA, Ball KK, Cruz NF (2007) A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem 102(2):466–478
Brown AM, Baltan Tekkok S, Ransom BR (2004) Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem Int 45(4):529–536. doi:10.1016/j.neuint.2003.11.005
Hertz L, Gibbs ME (2009) What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem 109(Suppl 1):10–16. doi:10.1111/j.1471-4159.2009.05939.x
Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry P-G, Moortele P-FVD, Gruetter R (2007) Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab 292(3):E946–E951
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2010) Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab 30(12):1895–1904. doi:10.1038/jcbfm.2010.151
Walz W (2004) Potassium homeostasis in the brain at the organ and cell level. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 595–609
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2010) Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab 30(3):586–602. doi:10.1038/jcbfm.2009.232
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27(11):1766–1791. doi:10.1038/sj.jcbfm.9600521
Walls AB, Heimburger CM, Bouman SD, Schousboe A, Waagepetersen HS (2009) Robust glycogen shunt activity in astrocytes: effects of glutamatergic and adrenergic agents. Neuroscience 158(1):284–292. doi:10.1016/j.neuroscience.2008.09.058
Shulman RG, Hyder F, Rothman DL (2001) Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA 98(11):6417–6422. doi:10.1073/pnas.101129298
Mangia S, Giove F, Tkac I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Ugurbil K (2009) Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab 29(3):441–463. doi:10.1038/jcbfm.2008.134
Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW (2004) Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305(5680):99–103. doi:10.1126/science.1096485
Pancani T, Anderson KL, Porter NM, Thibault O (2011) Imaging of a glucose analog, calcium and NADH in neurons and astrocytes: dynamic responses to depolarization and sensitivity to pioglitazone. Cell Calcium 50(6):548–558. doi:10.1016/j.ceca.2011.09.002
Hu Y, Wilson GS (1997) A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem 69(4):1484–1490
Mangia S, Garreffa G, Bianciardi M, Giove F, Di Salle F, Maraviglia B (2003) The aerobic brain: lactate decrease at the onset of neural activity. Neuroscience 118(1):7–10. doi:S0306452202007923
Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M (2004) Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. Eur J Neurosci 20(1):144–160
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281(Pt 1):21–40
Ellington WR (2001) Evolution and physiological roles of phosphagen systems. Annu Rev Physiol 63:289–325. doi:10.1146/annurev.physiol.63.1.289
da-Silva WS, Gomez-Puyou A, de Gomez-Puyou MT, Moreno-Sanchez R, de Felice FG, de Felice FG, de Meis L, Oliveira MF, Galina A (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 279(38):39846–39855. doi:10.1074/jbc.M403835200
Meyer LE, Machado LB, Santiago AP, da-Silva WS, De Felice FG, Holub O, Oliveira MF, Galina A (2006) Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem 281(49):37361–37371. doi:10.1074/jbc.M604123200
Wilson JE (2003) Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206(Pt 12):2049–2057
Beattie DS, Sloan HR, Basford RE (1963) Brain mitochondria. II. The relationship of brain mitochondria to glycolysis. J Cell Biol 19:309–316
Vallejo CG, Marco R, Sebastian J (1970) The association of brain hexokinase with mitochondrial membranes and its functional implications. Eur J Biochem 14(3):478–485
Land JM, Booth RF, Berger R, Clark JB (1977) Development of mitochondrial energy metabolism in rat brain. Biochem J 164(2):339–348
Belanger M, Magistretti PJ (2009) The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11(3):281–295
Bolanos JP, Almeida A (2010) The pentose-phosphate pathway in neuronal survival against nitrosative stress. IUBMB Life 62(1):14–18. doi:10.1002/iub.280
Dienel GA (2010) Astrocytes are ‘good scouts’: being prepared also helps neighboring neurons. J Cereb Blood Flow Metab 30(12):1893–1894. doi:10.1038/jcbfm.2010.152
Mangia S, DiNuzzo M, Giove F, Carruthers A, Simpson IA, Vannucci SJ (2011) Response to ‘comment on recent modeling studies of astrocyte-neuron metabolic interactions’: much ado about nothing. J Cereb Blood Flow Metab 31(6):1346–1353. doi:10.1038/jcbfm.2011.29
Ball S, Colleoni C, Cenci U, Raj JN, Tirtiaux C (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62(6):1775–1801. doi:10.1093/jxb/erq411
Hudson JW, Golding GB, Crerar MM (1993) Evolution of allosteric control in glycogen phosphorylase. J Mol Biol 234(3):700–721. doi:10.1006/jmbi.1993.1621
Hudson JW, Hefferon KL, Crerar MM (1993) Comparative analysis of species-independent, isozyme-specific amino-acid substitutions in mammalian muscle, brain and liver glycogen phosphorylases. Biochim Biophys Acta 1164(2):197–208
Roach PJ (2002) Glycogen and its metabolism. Curr Mol Med 2(2):101–120
Hardie DG, Carling D, Gamblin SJ (2011) AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci 36(9):470–477. doi:10.1016/j.tibs.2011.06.004
Field ML, Khan O, Abbaraju J, Clark JF (2006) Functional compartmentation of glycogen phosphorylase with creatine kinase and Ca2+ ATPase in skeletal muscle. J Theor Biol 238(2):257–268. doi:10.1016/j.jtbi.2005.05.017
Khakimova AK, Skolysheva LK, Shur SA, Vul’fson IL (1995) Interaction between glycogen phosphorylase b and creatinine kinase from rabbit skeletal muscle. Biokhimiia 60(2):278–288
Acknowledgments
We thank two anonymous referees for valuable comments and constructive suggestions to the manuscript. The author S. Mangia thanks the funding supports: Minnesota Medical Foundation, NIH grants BTRR-P41RR008079, P30 NS057091, NIH R01 DK62440. This publication was also supported by the NIH grant 1UL1RR033183 from the National Center for Research Resources (NCRR) to the University of Minnesota Clinical and Translational Science Institute (CTSI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Leif Hertz.
Rights and permissions
About this article
Cite this article
DiNuzzo, M., Mangia, S., Maraviglia, B. et al. The Role of Astrocytic Glycogen in Supporting the Energetics of Neuronal Activity. Neurochem Res 37, 2432–2438 (2012). https://doi.org/10.1007/s11064-012-0802-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0802-5