Abstract
Background–purpose
Totally extraperitoneal (TEP) endoscopic hernioplasty and Lichtenstein hernioplasty are the most commonly used approaches for inguinal hernia repair. However, current evidence on which is the preferred approach is inconclusive. This updated meta-analysis was conducted to track the accumulation of evidence over time.
Methods
Studies were identified by a systematic literature search of the EMBASE, PubMed, Cochrane Library, and Google Scholar databases. Fixed- and random-effects models were used to cumulatively assess the accumulation of evidence over time.
Results
The TEP cohort showed significantly higher rates of recurrences and vascular injuries compared to the Lichtenstein cohort; [Peto Odds ratio (OR) = 1.58 (1.22, 2.04), p = 0.005], [Peto OR = 2.49 (1.05, 5.88), p = 0.04], respectively. In contrast, haematoma formation rate, time to return to usual activities, and local paraesthesia were significantly lower in the TEP cohort compared to the Lichtenstein cohort; [Peto OR = 0.26 (0.16, 0.41), p ≤ 0.001], [mean difference = − 6.32 (− 8.17, − 4.48), p ≤ 0.001], [Peto OR = 0.26 (0.17, 0.40), p ≤ 0.001], respectively.
Conclusions
This study, which is based on randomised-controlled trials (RCTs) of high quality, showed significantly higher rates of recurrences and vascular injuries in the TEP cohort than in the Lichtenstein cohort. In contrast, rate of postoperative haematoma formation, local paraesthesia, and time to return to usual activities were significantly lower in the TEP cohort than in the Lichtenstein cohort. Future multicentre RCTs with strict adherence to the standards recommended in the Consolidated Standards of Reporting Trials guidelines will shed further light on the topic.
Similar content being viewed by others
Introduction
Inguinal hernia repair is the most common operation in general surgery with more than 20 million performed annually worldwide [1]. Most patients with an inguinal hernia are symptomatic and the treatment of choice is surgical repair with mesh using open or laparo-endoscopic approach. The use of mesh varies worldwide from 0 to 5% in low-resource countries to 95% in high-resource countries. The Swedish National registry reported that for the year 2015, the percentages of inguinal hernia repair techniques were as follows: Lichtenstein hernioplasty 64%, totally extraperitoneal (TEP) hernioplasty 25%, transabdominal preperitoneal (TAPP) hernioplasty 3%, open preperitoneal hernioplasty 3.3%, and tissue repair 0.8%. The German Herniamed registry reported the following data for the period from 2009 to 2016: TAPP hernioplasty 39%, TEP hernioplasty 25%, and Lichtenstein hernioplasty 24%. There is a lack of data from America and Asia [1].
Some possible complications of hernioplasty include recurrence necessitating reoperations in 10–15% of cases and chronic pain (lasting more than 3 months) in 10–12% of cases, which may lead to long-term disability [1].
To date, the evidence comparing TEP hernioplasty to Lichtenstein hernioplasty is non-conclusive [2, 3]. However, there has been new published evidence since the most recent meta-analysis. Therefore, we decided to perform an updated traditional and cumulative meta-analysis to estimate the impact of the new studies on the robustness of the statistical significance of existing meta-analyses comparing TEP hernioplasty and Lichtenstein hernioplasty. Recurrence rate and chronic persistent pain were selected as primary outcomes.
Methods
The preferred reporting items for systematic reviews and meta-analyses’ checklist was followed in this study [4].
Literature search
With the use of the search terms in the free text and Medical Subject Headings terms (“laparoscopic or endoscopic total extraperitoneal inguinal repair”, “laparoscopic or endoscopic total extraperitoneal inguinal hernioplasty”, “open with mesh Lichtenstein inguinal hernia repair”, “Lichtenstein’s technique”, “inguinal hernia repair with mesh”, “TEP”, “inguinal hernia”, or “randomised or randomized controlled trial”), a systematic search of literature published over the last 30 years was performed using the EMBASE, Medline (PubMed), Cochrane Library, and Google Scholar databases. A grey literature search was also performed in the clinicaltrials.gov website. References of the retrieved articles were checked manually for additional studies. Disagreements between the authors were resolved by consensus-based discussions.
Study selection, and inclusion and exclusion criteria
Only randomised-controlled trials (RCTs) that compared TEP laparoscopic inguinal hernia repair with Lichtenstein’s technique for inguinal hernia repair were included in this study. They fulfilled the following criteria: (1) clearly documented comparison of TEP laparoscopic approach and Lichtenstein’s technique for inguinal hernia repair, (2) report of at least one outcome measure, (3) inclusion of only the most recent publication in cases of multiple publications by the same institution, and (4) selection of TEP and Lichtenstein approaches from multi-arm RCTs.
Abstracts, retrospective studies, and non-English language publications were excluded from the analysis.
Data extraction and outcomes
Two reviewers (PG and NA) independently extracted the following summary data from the included studies: name of authors; year of publication; number of patients included in the TEP and Lichtenstein hernioplasty cohorts; duration of operation; conversion rate; rates of haematoma and seroma formation; incidences of wound infection, vascular injury, and visceral injury; time to return to usual activities; incidence of persisting pain or persisting numbness; and recurrence rate.
Definitions
Hernia recurrence was defined as any symptomatic or asymptomatic palpable lump or weakness in the operated groin found by the patient or the examining physician and exacerbated by the Valsalva manoeuvre. Chronic persisting pain was defined as pain of any severity (including testicular) persisting for more than 3 months after the operation. Impaired sensibility was defined as loss of the ability to register touch or the presence of numbness and tingling. Wound infection, vascular injury, and visceral injury were reported according to the definitions provided by the authors of the included studies. Operative time was defined as the time from the initial operative scalpel-to-skin contact to the placement of the last suture. Haematomas included wound and scrotal haematomas or ecchymoses but not bruising, and seromas included hydroceles. Time to return to usual activities was defined as the time taken to get back to normal social activities or work.
Statistical analysis
The methodological quality of all included RCTs was based on Cochrane’s criteria, which include random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and differences in baseline characteristics [5].
Statistical analysis was conducted using the STATA software (version 15, Stata Corp LP, College Station, TX, USA) and the Review Manager 5.3 software (Cochrane Collaboration, Oxford, England). Heterogeneity was assessed using the I2 test, and cut-off values of 25%, 50%, and 75% were considered of low, moderate, and high heterogeneity, respectively [6]. Where heterogeneity occurred, both fixed- and random-effects models were generated, and the conclusions compared, with the latter used where there were discrepancies. Fixed-effects models were used in cases of I2 value less than 25%.
Dichotomous variables were analysed based on odds ratios (ORs) with 95% confidence intervals. For the outcomes considered, the reference categories were selected, such that OR < 1 TEP.
Continuous variables were combined based on the mean difference (MD) and the standardised MD. The studies were then combined using the Mantel–Haenszel method in the first instance, with the Peto approach used when the cross-table has a zero cell [5, 6]. For studies that did not report the means and variances of the two groups, these values were estimated from the median, range, and the size of sample, using the technique described by Hozo et al. where possible [7].
In all analyses, the point estimate was considered significant at P < 0.05.
Sensitivity analysis
Analyses of both primary and secondary outcomes were calculated using the random-effects and fixed-effect models to assess the impact of heterogeneity on the robustness of the conclusions. Cumulative analysis was performed to track the accumulation of evidence and to determine if the results of the meta-analysis were dominated by a particular study [8].
Results
Search strategy and included study characteristics
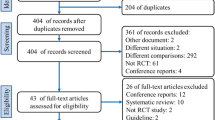
Twenty-one studies including 6573 patients were selected from a pool of 366 studies (Fig. 1). Of these patients, 3242 (49.3%) and 3331 (50.7%) underwent TEP and Lichtenstein hernioplasty, respectively [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Two abstracts, 1 in German and 1 in Spanish articles, were excluded. Non-significant differences were found in the demographic characteristics between the two cohorts (Table 1).
Quality assessment of included RCTs
The methodological quality of the RCTs was poor; only 4 of the 21 studies blinded participants and personnel, and one of them blinded the assessors of the outcomes (Table 2).
Primary outcomes
Recurrences
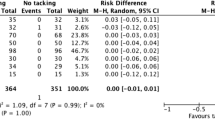
There was evidence of a higher recurrences in the TEP cohort (149/2678 patients; 6% of patients) compared with the Lichtenstein cohort (99/2790 patients; 4% of patients) [Peto OR = 1.58 (1.22, 2.04), p = 0.005] (Table 1, Fig. 2).
Chronic persistent pain
There was no difference in chronic pain between the TEP cohort (185/1617 patients; 11% of patients) and the Lichtenstein cohort (228/1862 patients; 13% of patients) [Peto OR = 0.81 (0.66, 1.00), p = 0.05] (Table 1).
Statistically significant secondary outcomes
There was evidence that the operative time was significantly shorter (by 11 min) in the Lichtenstein cohort than in the TEP cohort. Vascular injuries were significantly less in the Lichtenstein cohort than in the TEP cohort (Table 1).
There was evidence that the outcomes of haematoma formation rate, return to usual activities, and local paraesthesia were significantly better in the TEP cohort than in the Lichtenstein cohort (Table 1).
Statistically non-significant secondary outcomes
Non-significant differences were observed in the outcomes of seroma formation rate, incidence of wound infections, and time to discharge (Table 1).
Sensitivity analysis
Analysis of outcomes using fixed- and random-effects models did not reveal any discrepancies. Cumulative meta-analysis further supports the evidence that the recurrence rate was significantly lower in the Lichtenstein procedure. It depicts two periods one until 2004 where the differences were non-significant and the second which starts with the study of Neumayer in 2004 until the present day where the Lichtenstein repair demonstrates a significantly lower recurrence rate. Interestingly, the high-quality RCT by Gutlic [29] did not influence the results significantly either way (Fig. 3).
Discussion
This study shows that the TEP cohort had a higher recurrence rate and vascular injury rate than the Lichtenstein cohort. However, the TEP cohort had better outcomes in terms of haematoma formation rate, time to return to usual activities, and local paraesthesia compared with the Lichtenstein cohort. No difference was found between the 2 cohorts in terms of wound infection, persistent pain, and time to discharge.
The recurrence rate is difficult to explore, because it depends on varied follow-up periods [30]. The follow-up periods in this study also varied widely. Usually, the recurrence rate is estimated as twice the number of reoperations [31]. A Danish observational study reported that the reoperation rates following TEP laparoscopic hernioplasty and Lichtenstein hernioplasty were 3.3% and 2.4%, respectively [32]. It therefore suggests that the recurrence rates were around 6.6% and 4.8% for the laparoscopic and Lichtenstein hernioplasties, respectively. It is noteworthy that our study recorded recurrence rates of 6% and 4% for the TEP and Lichtenstein hernioplasties, respectively, which are similar to those of the above-mentioned study.
The reported incidence of clinically significant chronic persistent pain was 10–12% with a tendency to decrease with time [33, 34]. In this study, 11% of patients in the TEP cohort and 13% of the patients in the Lichtenstein cohort had chronic persistent pain, but no statistically significant differences were observed between the cohorts.
The reported incidence of vascular injury during inguinal hernioplasty is 0.1–0.4% [35]. Data from the German registry Herniamed reported significantly more vascular injuries of 1.39% in TEP compared to 1.13% in TAPP [36]. In this study, the rate of vascular injuries was significantly higher in the TEP cohort at 1.3% compared to 0.5% in the Lichtenstein cohort (Fig. 4, Table 1).
Furthermore, the haematoma formation rate was significantly less in the TEP cohort than in the open cohort. However, the lack of a haematoma severity classification and a common haematoma definition that is clinically relevant for the laparoscopic and open approaches make the extrapolation of objective conclusions difficult. Other potential contributors to diagnostic bias are preperitoneal haematomas that may be of similar sizes to superficial haematomas of open procedures, but may not be as easily diagnosable as those of the open procedure [1].
Cumulative meta-analysis further supports the findings of traditional meta-analysis by demonstrating that from 2004 until present, the recurrence rate is significantly lower for Lichtenstein repair. Interestingly, the most recently published high-quality RCT by Gutlic [29] did not influence the accumulated evidence.
To the best of the authors’ knowledge, this is the most up-to-date study and the first cumulative meta-analysis with 21 included studies and 6573 enrolled patients compared to the previous meta-analysis with 14 studies and 3279 patients [3]. However, the results of this study should be interpreted with caution due to the study limitations. The overall quality of the included RCTs was poor; only 4 of the 21 studies blinded the participants and the personnel, and one of them blinded the outcome assessors (Table 2). The total sample was quite heterogeneous as it included patients with primary, recurrent, and bilateral hernias. In addition, the studies were conducted in single centres and the follow-up periods varied widely (Table 3). Therefore, national and institutional characteristics, underpowered and heterogeneous samples, performance, and detection bias may have influenced the results. However, the outcome measures described in this study provide contemporaneous comparative data to allow surgeons to discuss the potential risks, benefits, and alternative treatment options with patients considering their options for inguinal hernia repair.
Implications for research
To shed further light on the topic, multicentre RCTs with the following characteristics should be conducted: strict adherence to standards recommended in the Consolidated Standards of Reporting Trials (CONSORT) guidelines; comparison of primary, recurrent, and simultaneously performed bilateral hernias in separate patient groups; adequate sample power with predefined outcome measures critical for decision making according to the Grading of Recommendations Assessment, Development, and Evaluation system (GRADE); blind outcome assessors (although we acknowledge that this would be very challenging when assessing longer term outcomes); common methods of outcome assessment; and a follow-up period at least 3 years [37, 38].
References
The Hernia Surge Group (2018) International guidelines for groin hernia management. Hernia 22:1–165
Koning GG, Wetterslev J, van Laarhoven CJHM et al (2013) The totally extraperitoneal method versus Lichtenstein’s technique for inguinal hernia repair: a systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. PLOS 8:e52599
Bobo Z, Nan W, Qin Q et al (2014) Meta-analysis of randomized controlled trials comparing Lichtenstein and totally extraperitoneal laparoscopic hernioplasty in treatment of inguinal hernias. J Surg Res 192:409–420
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(6):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Higgins JPT, Greens S (2011) Cochrane handbook for systematic reviews of interventions. Willey, Chichester (The Cochrane library issue 4)
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Hozo SP, Diulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Sterne JA (1998) Cumulative meta-analysis. Stata Tech Bull 42:13–16
Wright DM, Kennedy A, Baxter JN, Fullarton GM, Fife LM, Sunderland GT, O’Dwyer PJ (1996) Early outcome after open versus extraperitoneal endoscopic tension-free hernioplasty: A randomised trial. Surgery 119:552–557
Heikinen T, Haukipuro K, Hulko A (1998) A cost and outcome comparison between laparoscopic and Lichtenstein hernia operations in a day-case unit. Surg Endosc 12:1199–1203
Gokalp A, Inal M, Maralcan G, Baskonus I (2003) A prospective randomized study of Lichtenstein open tension-free versus laparoscopic totally extraperitoneal techniques for inguinal hernia repair. Acta Chir Belg 103:502–506
Andersson B, Hallén M, Leveau P, Bergenfelz A, Westerdahl J (2003) Laparoscopic extraperitoneal inguinal hernia repair versus open mesh repair: a prospective randomized controlled trial. Surgery 133:464–472
Colak T, Akca T, Kanik A, Aydin S (2003) Randomized clinical trial comparing laparoscopic totally extraperitoneal approach with open mesh repair in inguinal hernia. Surg Laparoscop Endosc Percutan Tech 13:191–195
Lal P, Kajla RK, Chander J, Saha R, Ramteke VK (2003) Randomized controlled study of laparoscopic total extraperitoneal versus open Lichtenstein inguinal repair. Surg Endosc 17:850–856
Bringman S, Ramel S, Heikkinen TJ, Englund T, Westman B, Anderberg B (2003) Tension-free inguinal hernia repair: tEP versus Mesh-plug versus Lichtenstein. A prospective randomized trial. Ann Surg 237:142–147
Neumayer L, Giobbie-Hurder A, Jonasson O, Fitzgibbons R, Dunlop D, Gibbs J, Reda D, Hendersen W (2004) Open mesh versus laparoscopic mesh repair of inguinal hernia. N Engl J Med 350:1819–1827
Heikkinen T, Bringman S, Ohtonen P, Kunelius P, Haukipuro K, Hulkko A (2004) Five –year outcome of laparoscopic and Lichtenstein hernioplasties. Surg Endosc 18:518–522
Lau H, Patil NG, Yuen WK (2006) Day-case endoscopic totally extraperitoneal inguinal hernioplasty versus open Lichtenstein hernioplasty for unilateral primary inguinal hernia in males. A randomized trial. Surg Endosc 20:76–81
Dedemadi G, Sgourakis J, Karaliotas C, Christofides T, Kouraklis G, Karaliotas C (2006) Comparison of laparoscopic and open tension-free repair of recurrent inguinal hernias: a prospective randomized study. Surg Endosc 20:1099–1104
Pokorny H, Klingler A, Schmid T, Fortely R, Hollinsky C, Kawji R, Steiner E, Pernthaler H, Függer R, Scheyer M (2008) Recurrence and complications after laparoscopic versus open inguinal hernia repair: results of a prospective randomized multicentre trial. Hernia 12:385–389
Hallén M, Bergenfelz A, Westerdahl J (2008) Laparoscopic extraperitoneal inguinal hernia repair versus open mesh repair: long-term follow-up of randomized controlled trial. Surgery 143:313–317
Hamza Y, Gabr E, Hammadi H, Khalil R (2010) Four-arm randomized trial comparing laparoscopic and open hernia repairs. Int J Surg 8:25–28
Eklund AS, Montgomery AK, Rasmussen C, Sandbue RP, Bergkvist LA, Rudberg CR (2009) Low recurrence rate after laparoscopic (TEP) and open (Lichtenstein) inguinal hernia repair. A randomized multicentre trial with 5-year follow-up. Ann Surg 249:33–38
Kuhia ST, Huttunen R, Silvasti SO, Heiskanen JT, Ahtola H, Uotila-Nieminen M et al (2009) Lichtenstein hernioplasty versus totally extraperitoneal laparoscopic hernioplasty in treatment of recurrent inguinal hernia- A prospective randomized trial. Ann Surg 249:384–387
Eker HH, Langeveld HR, Klitsie PJ, van’t Riet M, Stassen LP, Weidema WF, Steyerberg EW, Lange JF, Bonjer HJ, Jeekel J (2012) Randomized clinical trial of total extraperitoneal inguinal hernioplasty vs Lichtenstein repair: a long-term follow-up study. Arch Surg. 147:256–260
Dhankhar DS, Sharma N, Mishra T, Kaur N, Singh S, Gupta S (2014) Totally extraperitoneal repair under general anesthesia versus Lichtenstein repair under local anesthesia for unilateral inguinal hernia: a prospective randomized controlled trial. Surg Endosc 28:996–1002
Wang WJ, Chen JZ, Fang Q, Li JF, Jin PF, Li ZT (2013) Comparison of the effects of the laparoscopic hernia repair and Lichtenstein tension-free hernia repair. J Laparoendosc Adv Surg Tech 23:301–305
Moreno-Egea A (2014) Is it possible to eliminate sutures in open (Lichtenstein technique) and laparoscopic (totally extraperitoneal endoscopic) inguinal hernia repair? A randomized controlled trial with tissue adhesive (n-hexyl-a-cyanoacrylate). Surg Innov 21:590–599
Gutlic N, Gutlic A, Peterson U, Rogmark P, Montogomery A (2019) Ranodomized clinical trial comparing total extraperitoneal with Lichtenstein inguinal repair (TEPLICH trial). BJS 106:845–855
Staarink M, Van Veen RN, Hop WC, Weidema WF (2008) A 10-year repair follow-up study on endoscopic total extraperitoneal repair of primary and recurrent inguinal hernia. Surg Endosc 22:1803–1806
Sevonius D, Gunnarsson U, Nodin P, Nilsson E, Sandblom G (2011) Recurrent groin hernia surgery. Br J Surg 98:1489–1494
Burcharth J, Andresen K, Pommergaard H-C, Bisgaard T, Rosenberg J (2014) Recurrence patterns of direct and indirect inguinal hernias in a nationwide population in Denmark. Surgery 155:173–177
Alfieri S, Amid PK, Campanelli G, Izard G, Kehlet H, Wijsmuller AR, Di Miceli D, Doglietto GB (2011) International guidelines for prevention and management of post-operative chronic pain following inguinal hernia surgery. Hernia 15:239–249
Nienhuijs SW, Rosman C, Strobbe LJA, Wolff A, Bleichrodt RP (2008) An overview of the features influencing pain after inguinal hernia repair. Int J Surg 6:351–356
Spaw AT, Ennis BW, Spaw LP (1991) Laparoscopic hernia repair: the anatomic basis. J Laparosc Surg 1:269–277
Köckerling F, Bittner R, Jacob DA, Schug-Pass C, Laurenz C, Adolf D, Keller T, Stechemesser B (2015) TEP versus TAPP: comparison of the perioperative outcome in 17587 patients with primary unilateral inguinal hernia. Surg Endosc 29:3750–3760
Schulz KF, Altman DE, Moher D, CONSORT Group, CONSORT 2010 (2010) Updated guidelines for reporting parallel group randomized trials. PLos Med. 7:e1000251
Gyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ (2008) GRADE working group: what is “quality of evidence” and why is it important to clinicians? BMJ 336:995–998
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflicts of interest or financial ties to disclosure.
Ethical approval
This article did not require ethical approval of any kind.
Human and animal rights
This study does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent was not necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gavriilidis, P., Davies, R.J., Wheeler, J. et al. Total extraperitoneal endoscopic hernioplasty (TEP) versus Lichtenstein hernioplasty: a systematic review by updated traditional and cumulative meta-analysis of randomised-controlled trials. Hernia 23, 1093–1103 (2019). https://doi.org/10.1007/s10029-019-02049-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-019-02049-w