Abstract
An electrochemical aptamer-based assay is described for the determination of CFP-10 which is an early secretary biomarker of Mycobacterium tuberculosis. CFP-10 is specifically captured by its aptamer and then induces a DNA cross-linking click reaction, the release of CFP-10, and an amplification cycle of repeated CFP-10 release. This mechanism (with dual amplification via DNA click and target release cycle) causes more and more CFP-10 Apt strands on the electrode surface to expose their 5′ overhang and to hybridize with the DNA complexes linked to the gold nanoparticles (AuNPs). Consequently, large amounts of AuNPs, each loaded with a number of quadruplex DNA motifs, can be bound on the electrode surface and remarkably enhance the signal. Under optimal conditions, the method has a detection limit as low as 10 pg.mL−1 of CFP-10. The method was successfully applied to the diagnosis of M. tuberculosis in sputum.

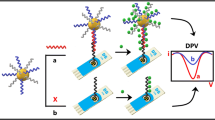
Schematic representation of an electrochemical CFP-10 (10-kDa culture filtrate protein) assay using click DNA cycling in combination with gold nanoparticles loaded with quadruplex DNA motifs. Click chemistry reaction between Dibenzocyclooctyne (DBCO)-DNA and azido-DNA can liberate the CFP-10 antigen for the next cycle, which can be viewed as the first amplification step. G-quadruplex-based DNAzyme is formed due to the guanine-rich sequences of DNA S1, which can be viewed as the second amplification step.







Similar content being viewed by others
References
Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R (2014) Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2(4):321–338
Sypabekova M, Bekmurzayeva A, Wang R, Li Y, Nogues C, Kanayeva D (2017) Selection, characterization, and application of DNA aptamers for detection of Mycobacterium tuberculosis secreted protein MPT64. Tuberculosis 104:70–78
Goletti D, Lee M-R, Wang J-Y, Walter N, Ottenhoff THM (2018) Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology 23(5):455–466. https://doi.org/10.1111/resp.13272
Floyd K, Glaziou P, Zumla A, Raviglione M (2018) The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the end TB era. Lancet Respir Med 6(4):299–314
Lei L, Liu Z, Zhang H, Yue W, Li CW, Yi C (2018) A point-of-need enzyme linked aptamer assay for Mycobacterium tuberculosis detection using a smartphone. Sensors Actuators B Chem 254:337–346
Liu W, Zou D, He X, Ao D, Su Y, Yang Z, Huang S, Zhao Q, Tang Y, Ma W (2018) Development and application of a rapidMycobacterium tuberculosisdetection technique using polymerase spiral reaction. Sci Rep 8(1):3003
Chen H, Liu F, Kwangnak L, Jaebeom Y, Zonghuang (2013) Sensitive detection of tuberculosis using nanoparticle-enhanced surface;plasmon resonance. Microchim Acta 180(5–6):431–436
Elsamadony H, Althani A, Tageldin MA, Azzazy HME (2017) Nanodiagnostics for tuberculosis detection. Expert Rev Mol Diagn 17(5):427–443
Kim J, Lee J, Lee KI, Park TJ, Kim HJ, Lee J (2013) Rapid monitoring of CFP-10 during culture of Mycobacterium tuberculosis by using a magnetophoretic immunoassay. Sensors Actuators B Chem 177(1):327–333
Troiano Araujo LDC, Wibrantz M, Rodriguez-Fernandez DE, Karp SG, Talevi AC, de Souza EM, Soccol CR, Thomaz-Soccol V (2019) Process parameters optimization to produce the recombinant protein CFP10 for the diagnosis of tuberculosis. Protein Expr Purif 154:118–125. https://doi.org/10.1016/j.pep.2018.09.016
Hong SC, Lee J, Shin HC, Kim CM, Park JY, Koh K, Kim HJ, Chang CL, Lee J (2011) Clinical immunosensing of tuberculosis CFP-10 in patient urine by surface plasmon resonance spectroscopy. Sensors Actuators B Chem 160(1):1434–1438
Makinen, J, Marjamaki, M, Marttila H, Soini H (2006) Evaluation of a novel strip test, GenoType Mycobacterium CM/AS, for species identification of mycobacterial cultures. Clin Microbiol Infect 12(5):481–483
Bai Y, Xue Y, Gao H, Wang L, Ding T, Bai W, Fan A, Zhang J, An Q, Xu Z (2008) Expression and purification of Mycobacterium tuberculosis ESAT-6 and MPT64 fusion protein and its immunoprophylactic potential in mouse model. Protein Expr Purif 59(2):189–196
Lambert L, Rajbhandary S, Qualls N, Budnick L, Catanzaro A, Cook S, Danielscuevas L, Reves EGR (2003) Costs of implementing and maintaining a tuberculin skin test program in hospitals and health departments. Infect Control Hosp Epidemiol 24(11):814–820
Sauzullo I, Massetti AP, Mengoni F, Rossi R, Lichtner M, Ajassa C, Vullo V, Mastroianni CM (2011) Influence of previous tuberculin skin test on serial IFN-Î3 release assays. Tuberculosis 91(4):322–326
Lee HJ, Choi HJ, Kim DR, Lee H, Jin JE, Kim YR, Lee MS, Cho SN, Kang YA (2016) Safety and efficacy of tuberculin skin testing with microneedle MicronJet600™ in healthy adults. Int J Tuberc Lung Dis 20(4):500–504
Sun J-R, Lee S-Y, Perng C-L, Lu J-J (2009) Detecting Mycobacterium tuberculosis in Bactec MGIT 960 cultures by Inhouse IS6110-based PCR assay in routine clinical practice. J Formos Med Assoc 108(2):119–125. https://doi.org/10.1016/s0929-6646(09)60042-5
Azmi UZM, Yusof NA, Kusnin N, Abdullah J, Suraiya S, Ong PS, Raston NHA, Abd Rahman SF, Fathil MFM (2018) Sandwich electrochemical Immunosensor for early detection of tuberculosis based on graphene/polyaniline-modified screen-printed gold electrode. Sensors 18(11). https://doi.org/10.3390/s18113926
He F, Xiong Y, Liu J, Tong F, Yan D (2016) Construction of au-IDE/CFP10-ESAT6 aptamer/DNA-AuNPs MSPQC for rapid detection of Mycobacterium tuberculosis. Biosens Bioelectron 77:799–804. https://doi.org/10.1016/j.bios.2015.10.054
Thakur H, Kaur N, Sareen D, Prabhakar N (2017) Electrochemical determination of M. tuberculosis antigen based on poly(3,4-ethylenedioxythiophene) and functionalized carbon nanotubes hybrid platform. Talanta 171:115–123
Torati SR, Reddy V, Yoon SS, Kim C (2016) Electrochemical biosensor for Mycobacterium tuberculosis DNA detection based on gold nanotubes array electrode platform. Biosens Bioelectron 78:483–488
Li J, Wang B, Gu S, Yang Y, Wang Z, Xiang Y (2017) Amperometric low potential aptasensor for the fucosylated Golgi protein 73, a marker for hepatocellular carcinoma. Microchim Acta 184(9):3131–3136. https://doi.org/10.1007/s00604-017-2334-9
Feng C, Wang Z, Chen T, Chen X, Mao D, Zhao J, Li G (2018) A dual-enzyme-assisted three-dimensional DNA walking machine using T4 polynucleotide kinase as activators and application in polynucleotide kinase assays. Anal Chem 90(4):2810–2815. https://doi.org/10.1021/acs.analchem.7b04924
Li J, Gao T, Gu S, Zhi J, Yang J, Li G (2017) An electrochemical biosensor for the assay of alpha-fetoprotein-L3 with practical applications. Biosens Bioelectron 87:352–357. https://doi.org/10.1016/j.bios.2016.08.071
Li C, Hu X, Lu J, Mao X, Xiang Y, Shu Y, Li G (2018) Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chem Sci 9(4):979–984. https://doi.org/10.1039/c7sc04663d
Zhanzhong MA, Yujiong W, Lianhua QIN, Yuansheng D, Qin XIE, Zhongyi HU (2008) Screening of aptamers to CFP-10 protein from Mycobacterium tuberculosis. Journal of Pathogen Biology 3(2):86–89
Tang X-L, Zhou Y-X, Wu S-M, Pan Q, Xia B, Zhang X-L (2014) CFP10 and ESAT6 aptamers as effective mycobacterial antigen diagnostic reagents. J Infect 69(6):569–580. https://doi.org/10.1016/j.jinf.2014.05.015
Zhang J, Liu Y, Lv J, Li G (2014) A colorimetric method for α-glucosidase activity assay and its inhibitor screening based on aggregation of gold nanoparticles induced by specific recognition between phenylenediboronic acid and 4-aminophenyl-α-d-glucopyranoside. Nano Res 8(3):920–930
Li J, He G, Wang B, Shi L, Gao T, Li G (2018) Fabrication of reusable electrochemical biosensor and its application for the assay of alpha-glucosidase activity. Anal Chim Acta 1026:140–146. https://doi.org/10.1016/j.aca.2018.04.015
Li J, He G, Mu C, Wang K, Xiang Y (2017) Assay of DNA methyltransferase 1 activity based on uracil-specific excision reagent digestion induced G-quadruplex formation. Anal Chim Acta 986:131–137. https://doi.org/10.1016/j.aca.2017.07.021
Yang D, Ning L, Gao T, Ye Z, Li G (2015) Enzyme-free dual amplification strategy for protein assay by coupling toehold-mediated DNA strand displacement reaction with hybridization chain reaction. Electrochem Commun 58:33–36. https://doi.org/10.1016/j.elecom.2015.06.001
Bakhori NM, Yusof NA, Abdullah J, Wasoh H, Noor SSM, Raston NHA, Mohammad F (2018) Immuno Nanosensor for the ultrasensitive naked eye detection of tuberculosis. Sensors 18(6). https://doi.org/10.3390/s18061932
Hong SC, Chen H, Lee J, Park H-K, Kim YS, Shin H-C, Kim C-M, Park TJ, Lee SJ, Koh K, Kim H-J, Chang CL, Lee J (2011) Ultrasensitive immunosensing of tuberculosis CFP-10 based on SPR spectroscopy. Sensors Actuators B Chem 156(1):271–275. https://doi.org/10.1016/j.snb.2011.04.032
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (Grant No. 81703088, and 81870695), the Medical Research Project of Jiangsu Provincial Health and Family Planning Commission, China (Grant No. H2018113), the Medical Science and Technology Development Foundation of Department of Health of Nanjing (YKK16107), and the Medical Science and Technology Foundation of Department of Health of Jiangsu Province (Grant No. Z2018034).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author declares that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 207 kb)
Rights and permissions
About this article
Cite this article
Li, J., Hu, K., Zhang, Z. et al. Click DNA cycling in combination with gold nanoparticles loaded with quadruplex DNA motifs enable sensitive electrochemical quantitation of the tuberculosis-associated biomarker CFP-10 in sputum. Microchim Acta 186, 662 (2019). https://doi.org/10.1007/s00604-019-3780-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3780-3