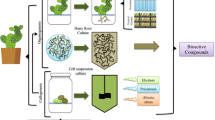
Abstract
Increased understanding of the interactions between endophytic fungi and plants has led to the discovery of a new generation of chemical compounds and processes between endophytic fungi and plants. Due to the long-term co-evolution between fungal endophytes and host plants, endophytes have evolved special biotransformation abilities, which can have critical consequences on plant metabolic processes and their composition. Biotransformation or bioconversion can impact the synthesis and decomposition of hormones, sugars, amino acids, vitamins, lipids, proteins, and various secondary metabolites, including flavonoids, polysaccharides, and terpenes. Endophytic fungi produce enzymes and various bioactive secondary metabolites with industrial value and can degrade or sequester inorganic and organic small molecules and macromolecules (e.g., toxins, pollutants, heavy metals). These fungi also have the ability to cause highly selective catalytic conversion of high-value compounds in an environmentally friendly manner, which can be important for the production/innovation of bioactive molecules, food and nutrition, agriculture, and environment. This work mainly summarized recent research progress in this field, providing a reference for further research and application of fungal endophytes.
Key points
•The industrial value of degradation of endophytes was summarized.
• The commercial value for the pharmaceutical industry is reviewed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial and fungal endophytes deeply involved in the physiology and metabolism of host plants can be found in almost all studied plants (Chen et al. 2020). Plant survival and development is often inseparable from the participation of such endophytes (Adamo et al. 2020). Therefore, plants should no longer be simply regarded as independent individuals; rather, they must be considered “symbiotic functional bodies” containing internal microorganisms (Kuzniar et al. 2020). In particular, endophytic fungi sustain part or their entire life cycle within healthy plants without causing any obvious diseases (Strobel 2018).
Of the 300,000 species of plants existing on earth, about one-sixth produce compounds potentially useful in disease treatment, able to synthesize various bioactive compounds, within the special internal environments in plants whose habitats are different from the ordinary environment (e.g., soil) for microorganisms (Nisa et al. 2015). These special habitats provide unique niches for a large number of endophytic fungi (Li et al. 2020a).
Among recent studies on secondary metabolites of fungi, 51% of newly discovered compounds with pharmacological activity have been found from endophytic fungi, with many showing versatile biological functions, including promoting plant nutrient absorption and helping plants cope with stress (Liu et al. 2020; Pilsyk et al. 2020; Xiao et al. 2020). They are also deeply involved in plant physiology and metabolism, including gene exchange, signal induction, and element sharing with plants. In addition, fungal secondary metabolites can be involved in regulating plant gene expression, modulating the activity and direction of branched metabolic pathways, and modifying plant metabolites and their production. With respect to the latter, fungal endophytes often impact the amount and concentration of (final) metabolites accumulating in plants tissues. In terms of the level of metabolic modifications, endophytic fungi can directly synthesize or decompose some metabolites; i.e., they can affect the metabolite composition of medicinal plants through biotransformation. For example, the endophytic fungi Flavobacterium sp. GE 32 and Arthritis sp. GE 17–18 in Panax ginseng can transform ginsenoside Rb1 that has low bioavailability into ginsenoside Rg3 and C-K (these products from ginseng root have been implicated in having a host of human health benefits), which has high bioavailability (Fu 2019; Fu et al. 2016).
Endophytic fungi complement the biotransformation capacity of the host plant, thus helping solve issues in complex compound production and the decomposition of difficult substances, such as industrial waste and pollutants. Biotransformation, including decomposition and synthesis affected by endophytic fungi both in vivo (in plant) and in vitro, is an area of significant active research. Recent advances have been successfully applied in drug synthesis (Louis et al. 2019), pollutant degradation, and food fermentation, e.g., wine brewing (Rho et al. 2020), thus providing opportunities for green and efficient solutions to industrial challenges. However, correlated summaries on these applications are largely lacking. The present study focuses on the biosynthesis and biodegradation abilities of endophytic fungi relevant to various applications, i.e., biotransformations and catalyses, to provide a scientific reference for sustainable production.
Research progress on the biodegradation activity of endophytic fungi
The research on and application of biodegradation activities of endophytic fungi includes the decomposition of small organic molecules and polymers.
Progress in degradation of small organic molecules by endophytic fungi
A balanced but potentially antagonistic relationship often exists between endophytic fungi and host plants (Schulz et al. 2015). Plants activate their defense system when many fungal endophytes initiate colonization, but the fungus often disrupt these defense responses by targeting plant defensive signaling factors to suppress host responses allowing for establishment within the plant more easily. For example, endophytic Mucor sp. KU234656 and Epichloë festucae KM400586, which have various hosts, decompose plant signaling molecules such as strigolactones (plant hormones that stimulate branching) and salicylic acid (plant hormones that regulate the plant immune system), to facilitate the penetration of the fungus into plant tissues (Rozpadek et al. 2018; Ambrose et al. 2015). Such fungal decomposing abilities are attributed to degradative enzyme systems, which include carbohydrate esterases, glycoside hydrolases, and polysaccharide lyases (Gramaje et al. 2020). Some endophytic fungi have evolved metabolic abilities to decompose plant-specific organic substances; for instance, Phomopsis liquidambari from the bark of Bischofia polycarpa can degrade sinapic acid (one of the most representative methoxy phenolic pollutants) to H2O and CO2 (Xie et al. 2016). Further to this, Burkholderia cenocepacia 869T2 from the roots of Vetiveria zizanioides has the unique ability to dechlorinate the compound dioxin (persistent carcinogenic byproducts of anthropogenic activities) into dibenzo-p-dioxin and subsequently decompose it into catechol and 2-hydroxysuccinate with low carcinogenicity (Nguyen et al. 2021). Endophytic fungi also develop various abilities to directly decompose defense substances. For example, Fusarium verticillioides from Zea mays, Acrmetonium sp. and F. moniliforme from Aphelundra tetragona, and Paecilomyces formosus HQ444388 from Glycine max can degrade toxic substances, such as benzoxazolin-2-(3H)-one (Schulz et al. 2016), aphelandrine (Christa et al. 1997), jasmonic acid (Bilal et al. 2018), 2-hydroxy-N-(2-hydroxypheyl) acetate (Zikmundova et al. 2002), 6-methoxy-benzox-azolin-2-one, and 2-benzoxazolinone (Glenn et al. 2016), in plants to adapt to the environment and establish a balanced symbiotic relationship with plants.
When an equilibrium is attained between an endophytic fungus and its host plant(s), a mutual relationship is established. Endophytic fungi can help plants avoid external damage through contributions of their unique biodegradation capability. For example, the endophytic fungus Neurospora intermedia MF362953 isolated from Saccharum officinarum can decompose phenylurea herbicide diuron [3-(3,4-dichlorophenyl)-1,1-dimethylurea] (Morais et al. 2017). Some endophytic fungi can degrade some host plants’ compounds, but the biochemical mechanism(s) of how these compounds are degraded has yet to be clearly elucidated. For instance, Paraconiothyrium variabilis LCP5644 from Cephalotaxus harringtonia and F. oxysporum 2T12J01A from Andrographis paniculata can decompose O-glycosides and change the metabolite profile of the host (Tian et al. 2014; Wang et al. 2014). Some endophytic fungi from Salvia miltiorrhiza can degrade limonene, geraniol, and pinene (plant essential oil components) into intermediates of terpenoid biosynthesis to produce new valuable biological products, and Mucor circinelloides DF20 from Salvia miltiorrhiza can promote tanshinone (pharmacological active component of host plant) biosynthesis and accumulation in Salvia miltiorrhiza root (Chen et al. 2018, 2021). However, the degradation of endophytic fungi can sometimes destroy the medicinal substances of plants. For example, Alternaria eureka 20131E1BL1 from Ruscus aculeatus can transform the spirochete alcohol skeleton of neoruscogenin, which used to treat chronic venous insufficiency, varicose veins, and hemorrhoids, into a cholesterol skeleton (Ozcinar et al. 2018), and endophytic fungal P. liquidambari from Bischofia polycarpa can completely decompose cinnamic acid (hepatoprotective agent) into CO2 and H2O, rendering it impossible for plants to synthesize flavonoids, thus reducing the active quality of medicinal plants (Xie and Dai 2015).
Endophytic fungi not only exhibit their degradation ability within the host plant but also display high degradation activity outside the plant. Nine endophytic fungi isolated from Plantago lanceolata, including Aspergillus niger, Eurotium repens, Leptosphaerulina chartatum, A. nidulans, E. amstelodami, Cladosporium pseudocladosporioides, Penicillium chrysogenum, Bipolaris sp., and Epicoccum nigrum, have been shown to be able to decompose non-steroidal anti-inflammatory drugs, such as diclofenac, diflunisal, ibuprofen, mefenamic, and piroxicam in vitro (Gonda et al. 2016). Endophytic fungi with unique biodegradation capabilities benefit from various enzymes co-evolved with host plants for a long time, particularly α-l-rhamnohydrolase, β-N-acetylhexosaminidase, and urease, which have industrial application values (Gramaje et al. 2020; Atmaca 2019). Many additives that are difficult to treat in the industry, such as reactive dark blue, reactive green, reactive turquoise blue, reactive brilliant red, reactive brilliant orange, triclosan, and malachite green (listed as a carcinogen by the Food and Drug Administration), are considered as common pollutants in the aquaculture industry (Zhou et al. 2018b). Three endophytic fungi, Myrothecium verrucaria DJTU-sh7, Glomerella sp., and Talaromyces stollii, isolated from Taxus chinensis can degrade reactive dark blue, reactive green, reactive black, reactive turquoise blue, reactive brilliant orange, and reactive brilliant red (refractory chemical dyes) (Hao et al. 2016). Klebsiella aerogenes M2017452 from Cyperus rotundus can degrade malachite green to nontoxic substances, e.g., N,N-dimethylaniline and 2-(4-dimethylamino-phenyl)-phenyl-methanone (Shang et al. 2019). Triclosan can be degraded into detoxifying metabolites, e.g., hydroquinone, (2Z,4E)-3-chloro-2,5-dihydroxyhexa-2,4-dienedioic acid, and (2Z,4E)-3-chloro-2,5-dihydroxyhexa-2,4-dienedial by Penicillium oxalicum FJ196840 isolated from Artemisia annua (Tian et al. 2018). In addition, endophytic fungi can be used to degrade organic substances, such as polycyclic aromatic hydrocarbon (Tardif et al. 2016), triphenylmethane (Gao et al. 2020a), cyanide (Al-Badri et al. 2020), azo compounds (Marzall-Pereira et al. 2019), and phenols (Rusanova et al. 2019), in industrial wastewater. For example, Trichoderma harzianum PTA-10317 from Taraxacum officinale L. can completely decompose phenanthrene (polycyclic aromatic hydrocarbon pollutant) into CO2 and H2O (Repas et al. 2017). Furthermore, endophytic fungus P. liquidambari from Oryza sativa can degrade more than 10 small molecule organic chemical pollutants, such as bisphenol, chloroalkane, chloroalkene, caprolactam, polyaromatic hydrocarbon, naphthalene, chlorochlorochlorohexane, chlorobenzene, aminobenzoate, styrene, fluorobenzoate, atrazine, dioxin, toluene, benzoate, and ethylbenzene (Zhou et al. 2017).
Progress in the degradation of organic polymers by endophytic fungi
Endophytic fungi have acquired the ability to decompose the aging cell wall and breakthrough various plant barriers in the process of establishing symbiosis with host plants by evolution. These interactions are aimed towards the fungus obtaining nutrients from the plant, but can also have the consequence of eliminating host “waste” byproducts, i.e., compounds that the plant cannot use, but may, in some instances, accumulate within plant tissues leading to toxicity (Suryanarayanan et al. 2012). In addition, fungal endoglucanases and cellobiohydrolases (endo- and exo-cellulases) can degrade cellulose and hemicellulose of plant for their invading or mutualistic symbiosis (Adamo et al. 2020). The unique biodegradation process of organic polymers in endophytic fungi often requires the assistance of redox system enzymes, such as lytic polysaccharide monooxygenases, ligninolytic peroxidases, laccase, and other enzymes produced by endophytic fungi, cellulose, and lignin as a major component of cell wall aging, which can be transformed into nutrients of endophytic fungi (Mathe et al. 2019). For example, Rickenella mellea JGI 334,780 from Alloclavaria purpurea can transform lignin, cellulose, hemicellulose, and lignin-like polymers in plant aging cell walls into their nutrients or help plant to dispose garbage (Korotkin et al. 2018). Endophytic fungi can produce endo-1,4-β-xylanase, xylan α-glucuronidase, acetylxylan esterase, and xylan acetylsterase to degrade xylan, while chitin can be degraded into nutrients by chitinase, polysaccharide lyase, and N-acetylglucosaminidase, all enzymes that can be produced by various endophytic fungi (Aranda-Martinez et al. 2016). For example, endophytic fungi Hymenoscyphus ericae and Pochonia chlamydosporia can decompose chitin from other invading microorganisms, fungal residual body, or soil into N-acetylglucosamine, thus providing an organic nitrogen source for plants (Kerley et al. 1995).
Endophytic fungal enzymes have been gradually used in industrial production, and the production of amylase, cellulase, laccase, lipase, protein, xylanase, pectinase, phytase, and phenoxidase has been matured and industrialized (Correa et al. 2014). A summary of the latest research results on endophytic fungal enzymes in the last 5 years is shown in Table 1.
With respect to industrial applications, a combination of the decomposition ability of endophytic fungi with physical and chemical pretreatment may reduce the loss of purely physical and chemical pretreatment. For example, in the sugar production industry, endophytic Ulocladium sp. from Eucalyptus Globus and F. verticillioides from Andropogon gayanus can be used to pretreat raw materials to improve the yield of sugar (de Almeida et al. 2019). The endophytic fungus Hypoxylon sp. CI-4 in T. distichum can transform cellulose into 1-acetyl-2-(1-hydroxyethyl)-cyclohexene, 2,3-dimethoxy-naphthalene, 2,5-furandione dihydro-3-methylene, and other organic substances with fuel value (Maxwell et al. 2018). Notably, the endophytic Chaetomium globosum CGMCC 6882 from a well-known folk medicinal plant Gynostemma pentaphyllum can successfully transform xanthan (a polymer containing β-1,4-glucosidic bond successfully linked to the main chain and a trisaccharide side-chain containing mannose, gluconic acid, and mannose) into a low-molecular-weight xanthan (LW-XG; the composition of LW-XG was glucose, mannose, and glucuronic acid at a molar ratio of 1.63:1.5:1.0) with antioxidant, anti-arthritis, anti-chondrocyte apoptosis, and anti-Staphylococcus aureus (Hu et al. 2019). These fungi also have a commercial value in environmental and industrial waste treatment. For example, the serine hydrolase secreted by Guignardia mangiferae E2702C and Zopfiella karachiensis E2719A can be used to treat synthetic material polyester polyurethane under anaerobic conditions (Russell et al. 2011).
Research progress on the biosynthesis of natural products by endophytic fungi
Endophytic fungi directly synthesize various natural products in plants
The reciprocal relationship between endophytic fungi and the host has been verified; however, many aspects of the complex co-evolution mechanisms that mediate these interactions remain unclear (Lu et al. 2019). Such co-evolutionary adaptations have been selected for the ability of endophytic fungi to produce signal substances that are either similar or different from the host, and endophytic fungi can provide new avenues for screening for efficient synthetic drugs, compounds useful in agriculture (plant growth promotion, protection from abiotic stress, protection from pathogens, etc.), food safety (harvest, post-harvest, storage), and other industrial applications (biofuels, bioplastics, etc.).
Endophytic fungi can produce various phytohormones, such as phytohormone indole-3-acetic acid, gibberellic acid, cytokinin, and phytoalexins, which were successively found from the endophytic fungi Serendipita indica, F. fujikuroi MI58289, and Piriformospora indica DSM11827 (Inaji et al. 2020; Niehaus et al. 2016; Li et al. 2016b). This finding indicated that endophytic fungi can participate in host signal regulation and affect host physiological and metabolic activities (Yuan et al. 2016; Bilal et al. 2018; Guarino et al. 2020).
Endophytic fungi can also synthesize some “simulated secondary metabolites” similar to or the same as host plants through “gene exchange” with host plants. The gene clusters mediating the synthesis of some of these “shared” metabolites have been proposed to be endophytic fungal origin, having been transferred to host plants through their long co-evolutionary history (Glenn et al. 2016). Currently, paclitaxel (an antineoplastic) (Shao et al. 2021), camptothecin (for antitumor) (Kaur et al. 2020), cinchonine (for treatment of malaria disease) (Maehara et al. 2011), and podophyllotoxin (inhibit herpes virus) (Vasundhara et al. 2016) can be synthesized by endophytic fungi and more than 90 high medicinal value metabolites (Archana et al. 2021). However, in other cases, the biosynthetic pathway mediating the synthesis of similar bioactive metabolites found in both endophytic fungi and their plant hosts has been found to be completely different. For example, the pathway for gibberellic acid (GA) biosynthesis of Gibberella fujikuroi IMI 58,289 is different from their host plants, and the fungal GAs is synthesized from acetyl-CoA via mevalonic acid pathway, but most plants, at least in the green parts, are predominantly produced by the methyl erythritol phosphate pathway (Bömke and Tudzynski 2009). The taxol (anticancer drug) biosynthetic pathway found in the endophytic fungus A. nidulans has low homology to the one reported for plant Taxus spp., suggesting that the taxol biosynthesis ability of this endophytic fungi may have evolved independently of the plant one (Elena et al. 2020).
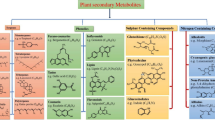
Some compounds synthesized by endophytic fungi are not made by host plant but are released into the tissues of host plant and can cause changes in the chemical composition of the host plant. For example, fungal ergot alkaloid and loline alkaloids can accumulate in plant tissues and which are important toxic substances to livestock (Fig. 1). These compounds were originally thought to be produced by the plant, Lolium perenne, until they were discovered to be exclusively produced by L. perenne endophytic E. festucae and Epichloë fungal species (Katrin et al. 2020; Panaccione et al. 2017). On the basis of these findings, scientists have reinoculated L. perenne with genetically modified Epichloë spp. as EAR1 and EAR37, in which production of the toxic alkaloids has been abolished, and leading to the elimination of the toxic substances in host plants, and improved quality of pasture production. These fungi are currently commercialized in Australia, North America, and other places (Qawasmeh et al. 2015, 2012). Another well studied and confirmed example is swainsonine, a toxin which can seriously poison livestock, and it also is one of the main bioactive chemicals in several Fabaceae plants, produced by endophytic Undifilum spp. and Alternaria spp., which were dominant fungal endophytes from Astragalus, Oxytropis, and Swainsona of Fabaceae plants (Moodley et al. 2019; Ren et al. 2017). A significant number of novel compounds with diverse activities continues to be found in various endophytic fungi, including flavonoids, alkaloids, and terpenoids (with main finds summarized in Table 2).
Important intermediates and end product of the loline alkaloid. (Asp, asparticacid; Asp4P, aspartic acid-4-phosphate; Asa, aspartyl-4-semialdehyde; Hse, homoserine; OAH, O-acetylhomoserine; P5C, pyrroline-5-carboxylate; Pro, proline; NL, norloline; NML, N-methylloline; NFL, N-formylloline). Double arrows indicate additional, non-illustrated intermediates
Highly selective catalytic activities of endophytic fungi
The use of endophytic fungi as a biocatalyst for the production of high-yield and high-purity compounds in an environmentally friendly manner has attracted significant research interest (Scalvenzi 2014). The catalysis and transformation of endophytic fungi have been mainly used for the following purposes: (i) overcoming the difficulties in chemical synthesis; (ii) improving the activity or reducing the toxicity of lead drugs; and (iii) assisting in the study of the structure–activity relationship of drugs (Özçinar et al. 2018).
Endophytic fungi can selectively catalyze the synthesis of O-glycoside and O-ether bonds. Endophytic Penicillium sp. JQ228238 from Polygonum cuspidatum can transform resveratrol into pterostilbene, which shows more metabolic stability and stronger anti-inflammatory and antioxidant activities (Xu et al. 2020), Epicoccum nigrum from Salix sp. can transform flavonoids into kaempferol-O-diglycide, which shows anticancer and antioxidant activity (Harwoko et al. 2019), and Neosartorya hiratsukae from Astragalus angustifolius is able to transform neoruscogenin into neoruscogenin-1-O-β-glucopyranoside, which is a potential leading compound with anti-inflammatory and anti-tumor activities (Özçinar et al. 2018). In addition, endophytic fungi can catalyze the synthesis of N-glycoside and amide bonds with high selectivity. For example, F. verticillioides from Zea mays catalyzed the formation of the N-glycosidic bond of carbamate to produce N-(2-hydroxyphenyl)-malonic acid with anticancer and antioxidant activity, and P. brasiliensis from Zea mays promoted the formation of an amide bond between halogenated benzoic acid and amino acid (Fill et al. 2018; Schulz et al. 2016). The most commercial potential of endophyte is highly regioselective oxidation to hydroxyl, carbonyl, and epoxy groups. Four endophytic fungi, P. oxalicum FJ196840, F. oxysporum, G. cingulata, and Umbelopsis isabellina FJ872076.1, from Senna spectabilis and Centella asiatica can catalyze the formation of the benzene ring in artemisinic acid (synthetic precursor of antimalarial drug artemisinin), carbonylation, diterpene ketation, enantioselective hydroxylation of (-)-(S)-propranolol (medicine for treating arrhythmia, angina pectoris, and hypertension), and artemisinic acid (Hao et al. 2018; Monteiro et al. 2017; Borges et al. 2009; Gao et al. 2015). Pestalotiopsis microspora JF487784 in Huperzia serrata can hydroxylate ursolic acid at special sites (Fu et al. 2011). In addition, similar bioconversion effects have been reported by endophytic fungi; for instance, Phomopsis sp. KY113119 and Neofusicoccum sp. MF276906 from Pinus sp. can efficiently catalyze ( +)-(R)-limonene to limonene-1,2-diol (Bier et al. 2017; Cecati et al. 2018), and endophytic Nodulisporium sp. JN254790 from Panax notoginseng can convert the carbon–carbon double bonds of ginsenosides Re to dihydroxy, forming a novel compound with antiplatelet aggregation activity, vinaginsenoside R13 (Luo et al. 2013). F. oxysporum from C. roseus can glycosylate vinblastine and finally produce vincristine with antitumor activity (Kumar et al. 2013). The redox reaction of endophytic fungi also has stereoselectivity. For example, P. crustosum and A. fumigatus DSM 21,023 from Viguiera robusta and Juniperus communis can catalyze highly enantioselective oxidation albendazole and deoxypodophyllotoxin to ( −)-albendazole sulfoxide (drug for treating cerebral cysticercosis) and podophyllotoxin, respectively (Carrao et al. 2011; Kusari et al. 2009). Four endophytic fungi, namely, N. parvum from Illicium verum and Bacillus megaterium, Pseudomonas sp., and P. chrysogenum from Raphanus sativus, were used for the stereoselective catalytic reduction of carbonyl group and the catalyzation of the reduction of acetophenone to (R)-1-phenylethanol and (S)-1-phenylethanol (Li et al. 2016a; Rodriguez et al. 2015). They can even catalyze specific regional chemical reactions. For instance, P. brasilianum from Melia azedarach can catalyze the Baeyer Villiger reaction regiochemistry of 1-indanone to produce two compounds: dihydrocoumarin and (-)-(R)-3-hydroxy-1-indanone (Fill et al. 2012).
One of the most important scientific applications of endophytic fungal catalytic activity is their use to assist in the study of the drug structure–activity relationships. For example, Penicillium sp. SWUKD4.1850 from the root of Aphelandra can catalyze the transformation of nigranoic acid (drugs for preventing cerebral ischemia–reperfusion injury) into new compounds with high biological activity (Qin et al. 2019). The endophytic Colletotrichum gloeosporioides and P. crustosum from Viguiera robusta, and Fusarium spp. from V. arenaria can all transform diketopiperazine to produce several antitumor diketopiperazine derivatives, such as (3R, 5aR, 6S, 10aR)-6-hydroxy-3-(hydroxymethyl)-2- methyl-3,10a-bis(methyl-thio)-2,3,5a,6,10,10a-hexahydro-pyrazino[1,2-α]indole-1,4-dione and 6-hydr- oxy-3-(hydroxymethyl)-2-methyl-3-(methylthio)-2,3,10,10a-tetrahydropy-razino[1,2-α]indole-1,4- dione (Guimaraes et al. 2010). The unique habitat of endophytic fungi makes them “micro-evolve” to some unique ability to synthesize certain novel skeleton compounds. For example, F. oxysporum ATCC MYA 4623 can catalyze hydrazine to form novel skeleton compounds with anti-inflammatory activity, 3-methyl-1,2,4-triazolo[3,4-α]phthalazine (Almeida et al. 2018). Two endophytic fungi, A. eureka 20131E1BL1 and N. hiratsukae 20131E2AR1-1 from Astragalus sp., can modify cycloastragenol and astragenol to produce new compounds 1–5 (Fig. 2) that have telomerase inhibitory effects and are expected to be used in anti-aging and anti-Alzheimer’s disease (Ekiz et al. 2019).
Conclusion and future perspectives
Although much of the research on endophytic fungi is still in its infancy, their biodegradation and biosynthesis capacity is receiving increasing research attention. Results from this research can have the potential to promote revolutionary developments of industries ranging from food safety and security to the discovery of novel biopharmaceutical compounds to understanding basic aspects of organismal interactions and evolution. However, some difficulties are still encountered in studies on endophytic fungi. These include:
-
(1) Lack of culture conditions: given the operational complexity of the plant internal environment and the often unique habitats of medicinal plants, although a large number of endophytic fungi have been detected using high-throughput sequencing, a significant number of endophytic fungi still cannot be effectively cultured in vitro.
-
(2) In vitro passage affects fungal physiology: For those fungi that can be cultured, in vitro passage often leads to decreasing activities of desired biological processes. Owing to the complexity of the interaction between endophytic fungi and their host plants and current limitations on the factors that mediate these interactions, in many instances, the biotransformation activity, efficiency, and desired product formation capabilities of many isolated endophytic fungi gradually decrease with increasing generations of subculturing on synthetic media, thus limiting potential commercialization efforts. As one example, the ability to synthesize camptothecin gradually declines in F. solani INFU/Ca/KF/3 because of the lack of its host C. acuminata continually providing stritosidine synthases in vitro (Kusari et al. 2011). Increasing our understanding and ability to manipulate these species interaction mechanisms is necessary.
-
(3) Poor understanding of the networks that mediate establishment and regulation of the fungal-plant interaction. Our current understanding of the factors that mediate host responses, fungal persistence, and (biochemical) pathway interactions remains limited. For example, the content of wihanolide A in Withania somnifera can be increased by 147% when infected with Sarocladium kiliense F800957 compared with those not infected (Ramesh et al. 2019). This regulatory mechanism also needs to be further elucidated.
Future directions:
-
(1) Although a lot of biotransforming activities have been found in plants, only a few of them are applied to mass production in real life. Thus, the future efforts should focus on strengthening the continuous industrial application research in vivo and in vitro.
-
(2) Application of high-throughput “omics” to the fungal endophyte-plant interactions. Use of high-throughput sequencing technology including transcriptomics, coupled to proteomics and metabolomics, should be applied to gain mechanistic insights into the degree of integration of fungal and plant genetic and biochemical networks. The application of information networks, artificial intelligence, and other disciplines, using network models to simulate the signal and material exchange and sharing of species interaction, should also be developed to study the biotransformation mechanisms of endophytic fungi.
-
(3) Continued screening and isolation of fungal endophytes and novel approaches at maintaining desired traits during in vitro culturing should be encouraged.
References
Adamo M, Chialva M, Calevo J, Rose S, Girlanda M, Perotto S, Balestrini R (2020) The dark side of orchid symbiosis: can Tulasnella calospora decompose host tissues? Int J Mol Sci 21:3139
Al-Badri BAS, Al-Maawali SS, Al-Balushi ZM, Al-Mahmooli IH, Al-Sadi AM, Velazhahan R (2020) Cyanide degradation and antagonistic potential of endophytic Bacillus subtilis strain BEB1 from Bougainvillea spectabilis Willd. All Life 13:92–98
Almeida MO, Lopes AA, Roberto PG, Bertoni BW, Pupo MT (2018) Unveiling the fungal biotransformation of hydralazine using 13C-precursor. Phytochem Lett 26:55–59
Ambrose KV, Tian Z, Wang Y, Smith J, Zylstra G, Huang B, Belanger FC (2015) Functional characterization of salicylate hydroxylase from the fungal endophyte Epichloe festucae. Sci Rep 5:10939
Amobonye A, Bhagwat P, Singh S, Pillai S (2021) Enhanced xylanase and endoglucanase production from Beauveria bassiana SAN01, an entomopathogenic fungal endophyte. Fungal Biol 125:39–48
Aranda-Martinez A, Lenfant N, Escudero N, Zavala-Gonzalez EA, Henrissat B, Lopez-Llorca LV (2016) CAZyme content of Pochonia chlamydosporia reflects that chitin and chitosan modification are involved in nematode parasitism. Environ Microbiol 18:4200–4215
Archana S, Dheeraj KS, Ravindra NK, James FW, Surendra KG (2021) Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: insights, avenues, and challenges. Microorganisms 9:197
Atmaca E (2019) The relationship between certain microbiological and some arbuscular mycorrhizal parameters of plants prevalent around an aluminum bauxite mine deposit. Appl Ecol Environ Res 17:10941–10961
Bao J, Li XX, He F, Zhang X, Zhu K, Tao H, Yu JH, Liu H, Zhang H (2020) Asperbutenolide A, an unusual aromatic butenolide dimer with diverse bioactivities from a marine-derived fungus Aspergillus terreus SCAU011. Tetrahedron Lett 61:152193
Ben MF, Frikha F, Daoud A, Chenari BA, Luptakova L, Alenezi FN, Al-Anzi BS, Oszako T, Gharsallah N, Belbahri L (2019) Response surface methodology optimization of an acidic protease produced by Penicillium bilaiae isolate TDPEF30, a newly recovered endophytic fungus from healthy roots of date palm trees (Phoenix dactylifera L.). Microorganisms 7:74
Bier MC, Medeiros AB, Soccol CR (2017) Biotransformation of limonene by an endophytic fungus using synthetic and orange residue-based media. Fungal Biol 121:137–144
Bilal S, Shahzad R, Khan AL, Kang SM, Imran QM, Al-Harrasi A, Yun BW, Lee IJ (2018) Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front Plant Sci 9:1273
Bömke C, Tudzynski B (2009) Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochemistry 70:1876–1893
Borges KB, Pupo MT, Bonato PS (2009) Enantioselective analysis of propranolol and 4-hydroxypropranolol by CE with application to biotransformation studies employing endophytic fungi. Electrophoresis 30:3910–3917
Cai J, Zhou XM, Yang X, Tang MM, Liao QY, Meng BZ, Liao S, Chen GY (2020) Three new bioactive natural products from the fungus Talaromyces assiutensis JTY2. Bioorg Chem 94:103362
Cai R, Jiang H, Zang Z, Li C, She Z (2019) New benzofuranoids and phenylpropanoids from the mangrove endophytic fungus, Aspergillus sp. ZJ-68. Marine Drugs 17:478
Carrao DB, Borges KB, Barth T, Pupo MT, Bonato PS, de Oliveira AR (2011) Capillary electrophoresis and hollow fiber liquid-phase microextraction for the enantioselective determination of albendazole sulfoxide after biotransformation of albendazole by an endophytic fungus. Electrophoresis 32:2746–2756
Castillo UF, Strobel GA, Mullenberg K, Condron MM, Teplow DB, Folgiano V, Gallo M, Ferracane R, Mannina L, Viel S, Codde M, Robison R, Porter H, Jensen J (2006) Munumbicins E-4 and E-5: novel broad-spectrum antibiotics from Streptomyces NRRL 3052. FEMS Microbiol Lett 255:296–300
Cecati FM, Magallanes-Noguera C, Tonn CE, Ardanaz CE, Kurina-Sanz M (2018) Ecofriendly chemical diversification of Eupatorium buniifolium essential oil by endophytic fungi. Process Biochem 64:93–102
Chen HM, Qi Y, He XY, Xu LN, Zhang WY, Lv XM, Zhang HH, Yang DF, Zhu YH, Liang ZS (2021) Endophytic fungus Mucor circinelloides DF20 promote tanshinone biosynthesis and accumulation in Salvia miltiorrhiza root. Plant Sci 307:110898
Chen WH, Wu SJ, Suna XL, Feng KM, Rahmand K, Tana HY, Yub LY, Lia TQ, Xu LC, Qin LP, Han T (2020) High-throughput sequencing analysis of endophytic fungal diversity in Cynanchum sp. S Afr J Bot 31:1–10
Chen H, Wu H, Yan B, Zhao H, Liu F, Zhang H, Sheng Q, Miao F, Liang Z (2018) Core microbiome of medicinal plant Salvia miltiorrhiza seed: a rich reservoir of beneficial microbes for secondary metabolism? Int J Mol Sci 19:672
Christa W, Orlando P, Manfred H (1997) Degradation of the polyamine alkaloid aphelandrine by endophytic fungi isolated from Aphelandra tetragona. FEMS Microbiol Lett I55:147–153
Cota BB, Tunes LG, Maia DNB, Ramos JP, Oliveira DM, Kohlhoff M, Alves TMA, Souza-Fagundes EM, Campos FF, Zani CL (2018) Leishmanicidal compounds of Nectria pseudotrichia, an endophytic fungus isolated from the plant Caesalpinia echinata (Brazilwood). Mem Inst Oswaldo Cruz 113:102–110
Correa RC, Rhoden SA, Mota TR, Azevedo JL, Pamphile JA, de Souza CG, Polizeli Mde L, Bracht A, Peralta RM (2014) Endophytic fungi: expanding the arsenal of industrial enzyme producers. J Ind Microbiol Biotechnol 41:1467–1478
da Silva P, de Souza M, Bianco E, da Silva S, Soares L, Costa E, da Silva F, Barison A, Forim M, Cass QB (2017) Antifungal polyketides and other compounds from amazonian endophytic Talaromyces Fungi. J Braz Chem Soc 29:622–630
de Almeida MN, Falkoski DL, Guimarães VM, de Rezende ST (2019) Study of gamba grass as carbon source for cellulase production by Fusarium verticillioides and its application on sugarcane bagasse saccharification. Ind Crop Prod 133:33–43
Dzoyem JP, Melong R, Tsamo AT, Maffo T, Kapche DGWF, Ngadjui BT, McGaw LJ, Eloff JN (2017) Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica. Rev Bras Farmacogn 27:251–253
Ekiz G, Yilmaz S, Yusufoglu H, Kirmizibayrak PB, Bedir E (2019) Microbial transformation of cycloastragenol and astragenol by endophytic fungi isolated from Astragalus species. J Nat Prod 82:2979–2985
Elena A, Georgios D, Peter P (2020) Bioactive secondary metabolites from endophytic fungi. Curr Med Chem 27:1836–1854
Fan B, Dewapriya P, Li F, Blumel M, Tasdemir D (2020) Pyrenosetins A-C, new decalinoylspirotetramic acid derivatives isolated by bioactivity-based molecular networking from the seaweed-derived fungus Pyrenochaetopsis sp. FVE-001. Mar Drugs 18:47
Felber AC, Specian V, Orlandelli RC, Costa AT, Polonio JC, Mourão KSM, Pamphile JA (2019) Endoglucanase production by endophytic fungi isolated from Vitis labrusca L. with peanut hull and sawdust as substrates. Biosci J 35:933–940
Fill TP, Pallini HF, Din ZU, Jurberg ID, da Silva JV, Rodrigues-Filho E (2018) Conjugation of antifungal benzoic acid derivatives as a path for detoxification in Penicillium brasilianum, an endophyte from Melia azedarach. Bioorg Chem 81:367–372
Fill TP, da Silva JV, de Oliveira KT, da Silva BF, Rodrigues-Fo E (2012) Oxidative potential of some endophytic fungi using 1-indanone as a substrate. J Microbiol Biotechnol 22:832–837
Forcina GC, Castro A, Bokesch HR, Spakowicz DJ, Legaspi ME, Kucera K, Villota S, Narvaez-Trujillo A, McMahon JB, Gustafson KR, Strobelt SA (2015) Stelliosphaerols A and B, sesquiterpene–polyol conjugates from an Ecuadorian fungal endophyte. J Nat Prod 78:3005–3010
Fu Y (2019) Biotransformation of ginsenoside Rb1 to Gyp-XVII and minor ginsenoside Rg3 by endophytic bacterium Flavobacterium sp. GE 32 isolated from Panax ginseng. Lett Appl Microbiol 68:134–141
Fu Y, Yin ZH, Wu LP, Yin CR (2016) Biotransformation of ginsenoside Rb1 to ginsenoside C-K by endophytic fungus Arthrinium sp GE 17–18 isolated from Panax ginseng. Lett Appl Microbiol 63:196–201
Fu SB, Yang JS, Cui JL, Meng QF, Feng X, Sun DA (2011) Multihydroxylation of ursolic acid by Pestalotiopsis microspora isolated from the medicinal plant Huperzia serrata. Fitoterapia 82:1057–1061
Gallo MBC, Chagas FO, Almeida MO, Macedo CC, Cavalcanti BC, Barros FWA, de Moraes MO, Costa-Lotufo LV, Pessoa C, Bastos JK, Pupo MT (2009) Endophytic fungi found in association with Smallanthus sonchifolius (Asteraceae) as resourceful producers of cytotoxic bioactive natural products. J Basic Microbiol 49:142–151
Gao SS, Li XM, Li CS, Proksch P, Wang BG (2011) Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg Med Chem Lett 21:2894–2897
Gao ZH, Dong XR, Gao RR, Sun DA (2015) Unusual microbial lactonization and hydroxylation of asiatic acid by Umbelopsis isabellina. J Asian Nat Prod Res 17:1059–1064
Gao TC, Qin D, Zuo S, Peng Y, Xu J, Yu B, Song H, Dong J (2020a) Decolorization and detoxification of triphenylmethane dyes by isolated endophytic fungus, Bjerkandera adusta SWUSI4 under non-nutritive conditions. Bioresour Bioprocess 7:53
Gao YQ, Du ST, Xiao J, Wang DC, Han WB, Zhang Q, Gao JM (2020b) Isolation and characterization of antifungal metabolites from the melia azedarach-associated fungus Diaporthe eucalyptorum. J Agri Food Chem 68:2418–2425
Chapla V, Zeraik M, Cafeu M, Silva G, Cavalheiro A, Bolzani V, Young M, Pfenning L, Araujo A (2018) Griseofulvin, diketopiperazines and cytochalasins from endophytic fungi Colletotrichum crassipes and Xylaria sp., and their antifungal, antioxidant and anticholinesterase activities. J Braz Chem Soc 29:1707–1713
Glenn AE, Davis CB, Gao M, Gold SE, Mitchell TR, Proctor RH, Stewart JE, Snook ME (2016) Two horizontally transferred xenobiotic resistance gene clusters associated with detoxification of benzoxazolinones by Fusarium Species. PLoS One 11:e0147486
Gonda S, Kiss-Szikszai A, Szucs Z, Balla B, Vasas G (2016) Efficient biotransformation of non-steroid anti-inflammatory drugs by endophytic and epiphytic fungi from dried leaves of a medicinal plant, Plantago lanceolata L. Int Biodeter Biodegr 108:115–121
Gramaje D, Berlanas C, Martinez-Diz MDP, Diaz-Losada E, Antonielli L, Beier S, Gorfer M, Schmoll M, Compant S (2020) Comparative genomic analysis of Dactylonectria torresensis strains from grapevine, soil and weed highlights potential mechanisms in pathogenicity and endophytic lifestyle. J Fungi (basel) 6:255–278
Guarino C, Marziano M, Tartaglia M, Prigioniero A, Postiglione A, Scarano P, Sciarrillo R (2020) Poaceae with PGPR bacteria and arbuscular mycorrhizae partnerships as a model system for plant microbiome manipulation for phytoremediation of petroleum hydrocarbons contaminated agricultural soils. Agronomy-Basel 10:547
Guimaraes DO, Borges WS, Vieira NJ, de Oliveira LF, da Silva CH, Lopes NP, Dias LG, Duran-Patron R, Collado IG, Pupo MT (2010) Diketopiperazines produced by endophytic fungi found in association with two Asteraceae species. Phytochemistry 71:1423–1429
Hao DC, Song SM, Mu J, Hu WL, Xiao PG (2016) Unearthing microbial diversity of Taxus rhizosphere via MiSeq high-throughput amplicon sequencing and isolate characterization. Sci Rep 6:22006
Hao T, YanJun M, WanYi L, JianWen W (2018) Efficient degradation of triclosan by an endophytic fungus Penicillium oxalicum B4. Environ Sci Pol Res 57:1–13
Harwoko H, Hartmann R, Daletos G, Ancheeva E, Frank M, Liu Z, Proksch P (2019) Biotransformation of host plant flavonoids by the fungal endophyte Epicoccum nigrum. ChemistrySelect 4:13054–13057
Hu X, Wang K, Yu M, He P, Qiao H, Zhang H, Wang Z (2019) Characterization and antioxidant activity of a low-molecular-weight xanthan gum. Biomolecules 9:730
Hussain H, Kock I, Al-Harrasi A, Al-Rawahi A, Abbas G, Green IR, Shah A, Badshah A, Saleem M, Draeger S, Schulz B, Krohn K (2014) Antimicrobial chemical constituents from endophytic fungus Phoma sp. Asian Pacific J Tropical Med 7:699–702
Ibrahim SRM, Asfour HZ (2018) Bioactive γ-butyrolactones from endophytic fungus Aspergillus versicolor. Int J Pharma 14:437–443
Inaji A, Okazawa A, Taguchi T, Nakamoto M, Katsuyama N, Yoshikawa R, Ohnishi T, Waller F, Ohta D (2020) Rhizotaxis modulation in arabidopsis is induced by diffusible compounds produced during the cocultivation of arabidopsis and the endophytic fungus Serendipita indica. Plant Cell Physiol 61:838–850
Jendželovská Z, Jendželovský R, Kuchárová B, Fedoročko P (2016) Hypericin in the light and in the dark: two sides of the same coin. Front Plant Sci 7:560
Kamel RA, Abdel-Razek AS, Hamed A, Ibrahim RR, Stammler HG, Frese M, Sewald N, Shaaban M (2020) Isoshamixanthone: a new pyrano xanthone from endophytic Aspergillus sp. ASCLA and absolute configuration of epiisoshamixanthone. Nat Prod Res 34:1080–1090
Katoch M, Salgotra A, Singh G (2014) Endophytic fungi found in association with Bacopa monnieri as potential producers of industrial enzymes and antimicrobial bioactive compounds. Braz Arch Biol Technol 57:714–722
Katrin GH, Wade JM, Catherine MM, Cory M, Alison JP (2020) Fungal alkaloid occurrence in endophyte-infected perennial ryegrass during seedling establishment. J Chem Ecol 46:410–421
Kaur P, Kumar V, Singh R, Dwivedi P, Dey A, Pandey DK (2020) Biotechnological strategies for production of camptothecin from fungal and bacterial endophytes. S Afr J Bot 134:135–145
Kerley SJ, Read DJ (1995) The biology of mycorrhiza in the ericaceae XVIII. chitin degradation by Hymenoscyphus ericae and transfer of chitin-nitrogen to the host plant. New Phytol 131:369–375
Khiralla A, Spina R, Varbanov M, Philippot S, Lemiere P, Slezack-Deschaumes S, André P, Mohamed I, Yagi SM, Laurain-Mattar D (2020) Evaluation of antiviral, antibacterial and antiproliferative activities of the endophytic fungus Curvularia papendorfii, and isolation of a new polyhydroxyacid. Microorganisms 8:1353
Korotkin HB, Swenie RA, Miettinen O, Budke JM, Chen KH, Lutzoni F, Smith ME, Matheny PB (2018) Stable isotope analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. Am J Bot 105:1869–1887
Krishnapura PR, Belur PD (2016) Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. J Mol Catal B-Enzym 124:83–91
Kumar A, Ahmad A (2013) Biotransformation of vinblastine to vincristine by the endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. Biocatal Biotransfor 31:89–93
Kusari S, Zühlke S, Spiteller M (2011) Effect of artificial reconstitution of the interaction between the plant Camptotheca acuminata and the fungal endophyte Fusarium solani on camptothecin biosynthesis. J Nat Prod 74:764–775
Kusari S, Lamshoft M, Spiteller M (2009) Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J Appl Microbiol 107:1019–1030
Kuzniar A, Wlodarczyk K, Grzadziel J, Wozniak M, Furtak K, Galazka A, Dziadczyk E, Skorzynska-Polit E, Wolinska A (2020) New insight into the composition of wheat seed microbiota. Int J Mol Sci 21:4636
Lai D, Wang A, Cao Y, Zhou K, Mao Z, Dong X, Tian J, Xu D, Dai J, Peng Y, Zhou LG, Liu Y (2016) Bioactive dibenzo-alpha-pyrone derivatives from the endophytic fungus Rhizopycnis vagum Nitaf 22. J Nat Prod 79:2022–2031
Lai D, Brotz-Oesterhelt H, Muller WEG, Wray V, Proksch P (2013) Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum. Fitoterapia 91:100–106
Li LY, Wang YD, Liu ZL, Sun BD, Yu M, Niu SB, Ding G (2020a) Resorcylic acid analogs from the desert plant endophytic fungus Rhinocladiella similis. Mycosystema 39:589–598
Li H, Zhang R, Cao F, Wang J, Hu Z, Zhang Y (2020b) Proversilins A-E, drimane-type sesquiterpenoids from the endophytic Aspergillus versicolor. J Nat Prod 83:2200–2206
Li HY, Li ZY, Ruan GH, Yu YK, Liu XG (2016a) Asymmetric reduction of acetophenone into R-(+)-1-phenylethanol by endophytic fungus Neofusicoccum parvum BYEF07 isolated from Illicium verum. Biochem Biophys Res Comm 473:874–878
Li L, Chen X, Ma C, Wu H, Qi S (2016b) Piriformospora indica requires kaurene synthase activity for successful plant colonization. Plant Physiol Biochem 102:151–160
Lin X, Ai W, Li M, Pang X, Ju Z, Guan D, Yang B, Zhou X, Wang J, Liu J, Wang LS, Liu YH (2020) Colletoindole A from the mangrove plant endophytic fungus Colletotrichum tropicale SCSIO 41022. Chem Biodivers 17:e1900040
Liu Z, Zhao JY, Sun SF, Li Y, Liu YB (2020) Fungi: outstanding source of novel chemical scaffolds. J Asian Nat Prod Res 22:99–120
Louis B, Sehrish I, Kiran N, Dobgima JF, Elsie LY, Robinson CJ, Yiboh MTN, Pranab R (2019) Biotechnological application of endophytic filamentous bipolaris and curvularia: a review on bioeconomy impact. World J Microbiol Biotechnol 35:69
Lu Y, Jing Z, Xixi Z, Junling S, Chunmei J, Dongyan S (2019) Beneficial effects of endophytic fungi colonization on plants. Appl Microbiol Biot 103:3327–3340
Luo YP, Song XP, Zheng CJ, Chen GY, Luo XX, Han JX (2020) Four new chromone derivatives from Colletotrichum gloeosporioides. Chem Biodivers 17:e1900547
Luo SL, Dang LZ, Li JF, Zou CG, Zhang KQ, Li GH (2013) Biotransformation of saponins by endophytes isolated from Panax notoginseng. Chem Biodivers 10:2021–2031
Ma KL, Dong SH, Li HY, Wei WJ, Tu YQ, Gao K (2021) Cytochalasins from Xylaria sp. CFL5, an endophytic fungus of Cephalotaxus fortunei. Nat Prod Bioprospect 11:87–98
Maehara S, Simanjuntak P, Kitamura C, Ohashi K, Shibuya H (2011) Cinchona alkaloids are also produced by an endophytic filamentous fungus living in cinchona plant. Chem Pharm Bull 59:1073–1074
Mahmoud MM, Abdel-Razek AS, Frese M, Soliman HSM, Sewald N, Shaaban M (2018) 3,4-Dihydro-quinolin-2-one derivatives from extremophilic Streptomyces sp. LGE21. Int J Pharma 27:1834–1842
Maroldi MMC, Vasconcellos VM, Lacava PT, Farinas CS (2018) Potential of mangrove-associated endophytic fungi for production of carbohydrolases with high saccharification efficiency. Appl Biochem Biotechnol 184:806–820
Marzall-Pereira M, Savi DC, Bruscato EC, Niebisch CH, Paba J, Aluizio R, Ferreira-Maba LS, Galli-Terasawa LV, Glienke C, Kava V (2019) Neopestalotiopsis species presenting wide dye destaining activity: report of a mycelium-associated laccase. Microbiol Res 228:126299
Mathe C, Fawal N, Roux C, Dunand C (2019) In silico definition of new ligninolytic peroxidase sub-classes in fungi and putative relation to fungal life style. Sci Rep 9:20373
Maxwell T, Blair RG, Wang Y, Kettring AH, Moore SD, Rex M, Harper JK (2018) A solvent-free approach for converting cellulose waste into volatile organic compounds with endophytic fungi. J Fungi 4:102
Monteiro MCP, Tavares DG, Nery EM, Queiroz MVd, Pereira OL, Cardoso PG (2020) Enzyme production by Induratia spp. isolated from coffee plants in Brazil. Braz Arch Biol Technol 63:e20180673
Monteiro AF, Seidl C, Severino VGP, Cardoso CL, Castro-Gamboa I (2017) Biotransformation of labdane and halimane diterpenoids by two filamentous fungi strains. R Soc Open Sci 4:170854
Moodley O, Sun Y, Sossah FL, Kakishima M, Pavlov IN, Li Y, Wang Q (2019) Application of toxigenic Alternaria oxytropis to soybeans and its effect on swainsonine detection in different environments. Bull Environ Contam Toxicol 102:268–274
Morais PV, Wang Y, Li H, Feng G, Du L, Zeng D (2017) Biodegradation of diuron by an endophytic fungus Neurospora intermedia DP8–1 isolated from sugarcane and its potential for remediating diuron-contaminated soils. Plos One 12:e0182556
Navada KK, Sanjeev G, Kulal A (2018) Enhanced biodegradation and kinetics of anthraquinone dye by laccase from an electron beam irradiated endophytic fungus. Int Biodeter Biodegr 132:241–250
Nguyen BAT, Hsieh JL, Lo SC, Wang SY, Hung CH, Huang E, Hung SH, Chin WC, Huang CC (2021) Biodegradation of dioxins by Burkholderia cenocepacia strain 869T2: Role of 2-haloacid dehalogenase. J Hazard Mater 401:123347
Niehaus EM, Munsterkotter M, Proctor RH, Brown DW, Sharon A, Idan Y, Oren-Young L, Sieber CM, Novak O, Pencik A, Tarkowska D, Hromadova K, Freeman S, Maymon M, Elazar M, Youssef SA, El-Shabrawy EM, Shalaby ABA, Houterman P, Brock NL, Burkhardt I, Tsavkelova EA, Dickschat JS, Galuszka P, Guldener U, Tudzynski B (2016) Comparative “omics” of the Fusarium fujikuroi Species complex highlights differences in genetic potential and metabolite synthesis. Genome Biol Evol 8:3574–3599
Nisa H, Kamili AN, Nawchoo IA, Shafi S, Shameem N, Bandh SA (2015) Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb Pathogenesis 82:50–59
Noriler SA, Savi DC, Ponomareva LV, Rodrigues R, Rohr J, Thorson JS, Glienke C, Shaaban KA (2019) Vochysiamides A and B: two new bioactive carboxamides produced by the new species Diaporthe vochysiae. Fitoterapia 138:104273
Noumeur SR, Teponno RB, Helaly SE, Wang XW, Harzallah D, Houbraken J, Crous PW, Stadler M (2020) Diketopiperazines from Batnamyces globulariicola, gen. & sp. nov. (Chaetomiaceae), a fungus associated with roots of the medicinal plant Globularia alypum in Algeria. Mycol Prog 19:589–603
Orlandelli RC, Santos MS, Polonio JC, Azevedo JL, Pamphile JA (2017) Use of agro-industrial wastes as substrates for α-amylase production by endophytic fungi isolated from Piper hispidum Sw. Acta Sci-Technol 39:255–261
Özçinar Ö, Tağ Ö, Yusufoglu H, Kivçak B, Bedir E (2018) Biotransformation of ruscogenins by Cunninghamella blakesleeana NRRL 1369 and neoruscogenin by endophytic fungus Neosartorya hiratsukae. Phytochemistry 152:1–9
Ozcinar O, Ozgur T, Yusufoglu H, Kivcak B, Bedir E (2018) Biotransformation of neoruscogenin by the endophytic fungus Alternaria eureka. J Nat Prod 81:1357–1367
Panaccione DG, Arnold SL (2017) Ergot alkaloids contribute to virulence in an insect model of invasive aspergillosis. Sci Rep 7:8930
Pilsyk S, Mieczkowski A, Golan MP, Wawrzyniak A, Kruszewska JS (2020) Internalization of the Aspergillus nidulans AstA transporter into mitochondria depends on growth conditions, and affects ATP levels and sulfite oxidase activity. Int J Mol Sci 21:7727
Qawasmeh A, Raman A, Wheatley W (2015) Volatiles in perennial ryegrass infected with strains of endophytic fungus: impact on African black beetle host selection. J Appl Entomol 139:94–104
Qawasmeh A, Obied HK, Raman A, Wheatley W (2012) Influence of fungal endophyte infection on phenolic content and antioxidant activity in grasses: interaction between Lolium perenne and different strains of Neotyphodium lolii. J Agric Food Chem 60:3381–3388
Qin D, Shen W, Wang J, Han M, Chai F, Duan X, Yan X, Guo J, Gao T, Zuo SH, Dong JY (2019) Enhanced production of unusual triterpenoids from Kadsura angustifolia fermented by a symbiont endophytic fungus, Penicillium sp. SWUKD4.1850. Phytochemistry 158:56–66
Ramesh KK, Sucheta S, Shiv SP, Alok KCS, Vivek B (2019) Fungal endophytes attune withanolide biosynthesis in Withania somnifera, prime to enhanced withanolide A content in leaves and roots. World J Microb Biot 35:20
Reddy P, Elkins A, Hemsworth J, Guthridge K, Vassiliadis S, Read E, Spangenberg G, Rochfort S (2020) Identification and distribution of novel metabolites of lolitrem B in mice by high-resolution mass spectrometry. Molecules 25:372
Ren Z, Song R, Wang S, Quan H, Yang L, Sun L, Zhao B, Lu H (2017) The biosynthesis pathway of swainsonine, a new anticancer drug from three endophytic fungi. J Microbiol Biotechnol 27:1897–1906
Repas TS, Gillis DM, Boubakir Z, Bao X, Samuels GJ, Kaminskyj SGW (2017) Growing plants on oily, nutrient-poor soil using a native symbiotic fungus. PLoS One 12:e0186704
Rho H, Van Epps V, Kim SH, Doty SL (2020) Endophytes increased fruit quality with higher soluble sugar production in honeycrisp apple (Malus pumila). Microorganisms 8:2076–2607
Rodriguez JPG, Bernardi DI, Gubiani JR, Magalhaes OJ, Morais URP, Bertonha AF, Bandeira KF, Bulla JIQ, Sette LD, Ferreira AG, Batista JM, Silva TD, dos Santos RA, Martins CHG, Lira SP, da Cunha MG, Trivella DBB, Grazzia N, Gomes NES, Gadelha F, Miguel DC, Cauz ACG, Brocchi M, Berlinck RGS (2020) Water-soluble glutamic acid derivatives produced in culture by Penicillium solitum IS1-A from king george island, maritime antarctica. J Nat Prod 83:55–65
Rodriguez P, Magallanes NC, Menendez P, Orden AA, Gonzalez D, Kurina SM, Rodriguez S (2015) A study of Raphanus sativus and its endophytes as carbonyl group bioreducing agents. Biocatal Biotransfor 33:121–129
Rozpadek P, Domka AM, Nosek M, Wazny R, Jedrzejczyk R, Wiciarz M, Turnau K (2018) The role of strigolactone in the cross-talk between Arabidopsis thaliana and the endophytic fungus Mucor sp. Front Microbiol 9:441
Rusanova M, Rusanov K, Butterweck V, Atanassov I (2019) Exploring the capacity of endophytic fungi isolated from medicinal plants for fermentation and phenolics biotransformation of rose oil distillation wastewater. Biotechnol Biotec Eq 33:651–663
Russell JR, Huang J, Anand P, Kucera K, Sandoval AG, Dantzler KW, Hickman D, Jee J, Kimovec FM, Koppstein D, Marks DH, Mittermiller PA, Nunez SJ, Santiago M, Townes MA, Vishnevetsky M, Williams NE, Vargas MPN, Boulanger LA, Bascom-Slack C, Strobel SA (2011) Biodegradation of polyester polyurethane by endophytic fungi. Appl Environ Microbiol 77:6076–6084
Santiago C, Sun L, Munro MHG, Santhanam J (2014) Polyketide and benzopyran compounds of an endophytic fungus isolated from Cinnamomum mollissimum: biological activity and structure. Asian Pacific J Tropical Biomed 4:627–632
Savi DC, Noriler SA, Ponomareva LV, Thorson JS, Rohr J, Glienke C, Shaaban KA (2020) Dihydroisocoumarins produced by Diaporthecf. heveae LGMF1631 inhibiting citrus pathogens. Folia Microbiol 65:381–392
Scalvenzi L (2014) New frontiers of essential oils research: biotransformation of the phytocomplex and its pure compounds by endophytic fungi. International Symposium on Medicinal Plants and Natural Products 1030:125–132
Schafhauser T, Jahn L, Kirchner N, Kulik A, Flor L, Lang A, Caradec T, Fewer DP, Sivonen K, van Berkel WJH, Jacques P, Weber T, Gross H, van Pee KH, Wohlleben W, Ludwig-Muller J (2019) Antitumor astins originate from the fungal endophyte Cyanodermella asteris living within the medicinal plant Aster tataricus. Proc Natl Acad Sci USA 116:26909–26917
Schulz M, Filary B, Kuehn S, Colby T, Harzen A, Schmidt J, Sicker D, Hennig L, Hofmann D, Disko U, Anders N (2016) Benzoxazolinone detoxification by N-glucosylation: the multi-compartment-network of Zea mays L. Plant Signal Behav 11:e1119962
Schulz B, Haas S, Junker C, Andree N, Schobert M (2015) Fungal endophytes are involved in multiple balanced antagonisms. Curr Sci 109:39–45
Selim K, Elkhateeb W, Tawila A, El-Beih A, Abdel-Rahman T, El-Diwany A, Ahmed E (2018) Antiviral and antioxidant potential of fungal endophytes of Egyptian medicinal plants. Fermentation 4:49
Shang N, Ding M, Dai M, Si H, Li S, Zhao G (2019) Biodegradation of malachite green by an endophytic bacterium Klebsiella aerogenes S27 involving a novel oxidoreductase. Appl Microbiol Biotechnol 103:2141–2153
Shao FJ, Wilson IW, Qiu DY (2021) The research progress of taxol in Taxus. Curr Pharm Biotechnol 22:360–366
Shao Y, Yan H, Yin T, Sun Z, Xie H, Song L, Sun K, Li W (2020) New azaphilones from Penicillium variabile, a fungal endophyte from roots of Aconitum vilmorinianum. J Antibiot 73:77–81
Silva FA, Liotti RG, Boleti APA, Reis EM, Passos MBS, Dos Santos EL, Sampaio OM, Januario AH, Branco CLB, Silva GF, de Mendonca EAF, Soares MA (2018) Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS One 13:e0195874
Strobel G (2018) The emergence of endophytic microbes and their biological promise. J Fungi 2:57
Sun X, Wang G, Xiao H, Jiang J, Xiao D, Xing B, Li A, Zhang Y, Sun K, Xu Y, Guo L, Yang D, Ma M (2020) Strepimidazoles A-G from the plant endophytic Streptomyces sp. PKU-EA00015 with inhibitory activities against a plant pathogenic fungus. J Nat Prod 83:2246–2254
Sujarit K, Mori M, Dobashi K, Shiomi K, Pathom-Aree W, Lumyong S (2020) New antimicrobial phenyl alkenoic acids isolated from an oil palm rhizosphere-associated actinomycete, Streptomyces palmae CMU-AB204(T). Microorganisms 8:350
Supaphon P, Preedanon S (2019) Evaluation of in vitro alpha-glucosidase inhibitory, antimicrobial, and cytotoxic activities of secondary metabolites from the endophytic fungus, Nigrospora sphaerica, isolated from Helianthus annuus. Ann Microbiol 69:1397–1406
Suryanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Gopalan V (2012) Fungal endophytes: an untapped source of biocatalysts. Fungal Divers 54:19–30
Tardif S, Yergeau E, Tremblay J, Legendre P, Whyte LG, Greer CW (2016) The willow microbiome is influenced by soil petroleum-hydrocarbon concentration with plant compartment-specific effects. Front Microbiol 7:1363
Tawfike AF, Romli M, Clements C, Abbott G, Young L, Schumacher M, Diederich M, Farag M, Edrada-Ebel R (2019) Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. J Chromatogr B Analyt Technol Biomed Life Sci 1106–1107:71–83
Tian H, Ma YJ, Li WY, Wang JW (2018) Efficient degradation of triclosan by an endophytic fungus Penicillium oxalicum B4. Environ Sci Pol Res 25:8963–8975
Tian Y, Amand S, Buisson D, Kunz C, Hachette F, Dupont J, Nay B, Prado S (2014) The fungal leaf endophyte Paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry 108:95–101
Toghueo RMK, Zabalgogeazcoa I, Vázquez de Aldana BR, Boyom FF (2017) Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S Afr J Bot 109:146–153
Vasundhara M, Kumar A, Reddy MS (2016) Molecular approaches to screen bioactive compounds from endophytic fungi. Front Microbiol 7:1774
Wang A, Yin R, Zhou Z, Gu G, Dai J, Lai D, Zhou L (2020) Eremophilane-type sesquiterpenoids from the endophytic fungus Rhizopycnis vagum and their antibacterial, cytotoxic, and phytotoxic activities. Front Chem 8:596889
Wang Y, Li Y, Li S, Li Q, Fan W, Kiatoukosin L, Chen J (2019) Extracellular polysaccharides of endophytic fungus Alternaria tenuissima F1 from Angelica sinensis: Production conditions, purification, and antioxidant properties. Int J Biol Macromol 133:172–183
Wang JH, Wang ZT, Wang LL, Wang ZJ, Ma Z, Chou GX, Hu ZB, Li WK (2014) Biotransformation of neoandrographolide by endophytic fungus from Dendrobium officinale Kimuraet Migo. Asian J Chem 26:3457–3460
Wang HL, Wen K, Zhao XY, Wang XD, Li AY, Hong HZ (2009) The inhibitory activity of endophytic Bacillus sp. strain CHM1 against plant pathogenic fungi and its plant growth-promoting effect. Crop Prot 28:634–369
Xiao X, Wei C, Xin L, Heng Y, Guitai W, Kunming D, Xiuliang T, Lanfang Y, Youpin W, Yadong L, Haoshuang G, Xingguo W (2020) Selenate reduction and selenium enrichment of tea by the endophytic Herbaspirillum sp. strain WT00C. Curr Microbiol 77:588–601
Xie XG, Huang CY, Fu WQ, Dai CC (2016) Potential of endophytic fungus Phomopsis liquidambari for transformation and degradation of recalcitrant pollutant sinapic acid. Fungal Biol 120:402–413
Xie XG, Dai CC (2015) Biodegradation of a model allelochemical cinnamic acid by a novel endophytic fungus Phomopsis liquidambari. Int Biodeter Biodegr 104:498–507
Xu Z, Tian J, Gan L, Tian Y (2020) Discovery of the endophytic fungi from Polygonum cuspidatum and biotransformation of resveratrol to pterostillbene by the endophyte Penicillium sp. F5. Appl Biochem Microbiol 56:313–320
Yan Z, Wen S, Ding M, Guo H, Huang C, Zhu X, Huang J, She Z, Long Y (2019) The purification, characterization, and biological activity of new polyketides from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002. Mar Drugs 17:414
Yang X, Wu P, Xue J, Li H, Wei X (2020a) Cytochalasans from endophytic fungus Diaporthe sp. SC-J0138. Fitoterapia 145:1046
Yang ZJ, Zhang YF, Wu K, Xu YX, Meng XG, Jiang ZT, Ge M, Shao L (2020b) New azaphilones, phomopsones A-C with biological activities from an endophytic fungus Phomopsis sp. CGMCC No. 5416. Fitoterapia 145:1045
Yu X, Muller WEG, Meier D, Kalscheuer R, Guo Z, Zou K, Umeokoli BO, Liu Z, Proksch P (2020) Polyketide derivatives from mangrove derived endophytic fungus Pseudopestalotiopsis theae. Mar Drugs 18:129
Yu WG, He Y, Chen YF, Gao XY, Ning WE, Liu CY, Tang TF, Liu Q, Huang XC (2019) Fumigaclavine C attenuates adipogenesis in 3T3-L1 adipocytes and ameliorates lipid accumulation in high-fat diet-induced obese mice. Korean J Physiol Pha 23:161–169
Yuan X, Wang XF, Xu K, Li W, Chen D, Zhang P (2020) Characterization of a new insecticidal anthraquinone derivative from an endophyte of Acremonium vitellinum against Helicoverpa armigera. J Agric Food Chem 68:11480–11487
Yuan J, Zhou J-Y, Li X, Dai CC (2016) The primary mechanism of endophytic fungus Gilmaniella sp. AL12 promotion of plant growth and sesquiterpenoid accumulation in Atractylodes lancea. Plant Cell Tiss Org 125:571–584
Zaferanloo B, Bhattacharjee S, Ghorbani MM, Mahon PJ, Palombo EA (2014) Amylase production by Preussia minima, a fungus of endophytic origin: optimization of fermentation conditions and analysis of fungal secretome by LC-MS. BMC Microbiol 14:15
Zhang H, Wang X, Li R, Sun X, Sun S, Li Q, Xu C (2017) Preparation and bioactivity of exopolysaccharide from an endophytic fungus Chaetomium sp. of the medicinal plant Gynostemma Pentaphylla. Pharmacogn Mag 13:477–482
Zhao M, Guo DL, Liu GH, Fu X, Gu YC, Ding LS, Zhou Y (2020) Antifungal halogenated cyclopentenones from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain-many compounds strategy. J Agric Food Chem 68:185–192
Zhou J, Li X, Chen Y, Dai CC (2017) De novo transcriptome assembly of Phomopsis liquidambari provides insights into genes associated with different lifestyles in rice (Oryza sativa L.). Front Plant Sci 8:121
Zhou QY, Yang XQ, Zhang ZX, Wang BY, Hu M, Yang YB, Zhou H, Ding ZT (2018a) New azaphilones and tremulane sesquiterpene from endophytic Nigrospora oryzae cocultured with Irpex lacteus. Fitoterapia 130:26–30
Zhou X, Zhang J, Pan Z, Li D (2018b) Review of methods for the detection and determination of malachite green and leuco-malachite green in aquaculture. Crit Rev Anal Chem 14:1–20
Zhu P, Bu H, Tan S, Liu J, Yuan B, Dong G, Wang M, Jiang Y, Zhu H, Li H, Li ZJ, Jiang JH, Wu M, Li RP (2020) A novel cochlioquinone derivative, CoB1, regulates autophagy in Pseudomonas aeruginosa infection through the PAK1/Akt1/mTOR signaling pathway. J Immunol 205:1293–1305
Zhu X, Chen J, Zhu S, He Y, Ding W, Li C (2017) Two new compounds from Nigrospora sphaerica ZMT05, a fungus derivated from Oxya chinensis Thunber. Nat Prod Res 32:2375–2381
Zikmundova M, Drandarov K, Bigler L, Hesse M, Werner C (2002) Biotransformation of 2-benzoxazolinone and 2-hydroxy-1,4-benzoxazin-3-one by endophytic fungi isolated from Aphelandra tetragona. Appl Environ Microbiol 68:4863–4870
Funding
This work was financially supported by Shanxi Scholarship Council of China (No. 2020–013) and the National Natural Science Foundation of China (No. 31670328).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.L. and J.L.C.; writing—original draft preparation, X.L.; writing—review and editing, J.L.C. and Z.Y.Z.; supervision, M.L.W. and J.L.W. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Zhou, ZY., Cui, JL. et al. Biotransformation ability of endophytic fungi: from species evolution to industrial applications. Appl Microbiol Biotechnol 105, 7095–7113 (2021). https://doi.org/10.1007/s00253-021-11554-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11554-x