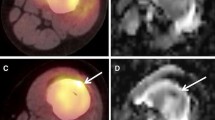
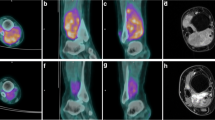
Abstract
Purpose
The objective of this study was to evaluate positron emission tomography (PET) using 18F-fluoro-2-deoxy-D-glucose (FDG) in comparison to volumetry and standardized magnetic resonance imaging (MRI) parameters for the assessment of histological response in paediatric bone sarcoma patients.
Methods
FDG PET and local MRI were performed in 27 paediatric sarcoma patients [Ewing sarcoma family of tumours (EWS), n = 16; osteosarcoma (OS), n = 11] prior to and after neoadjuvant chemotherapy before local tumour resection. Several parameters for assessment of response of the primary tumour to therapy by FDG PET and MRI were evaluated and compared with histopathological regression of the resected tumour as defined by Salzer-Kuntschik.
Results
FDG PET significantly discriminated responders from non-responders using the standardized uptake value (SUV) reduction and the absolute post-therapeutic SUV (SUV2) in the entire patient population (∆SUV, p = 0.005; SUV2, p = 0.011) as well as in the subgroup of OS patients (∆SUV, p = 0.009; SUV2, p = 0.028), but not in the EWS subgroup. The volume reduction measured by MRI/CT did not significantly discriminate responders from non-responders either in the entire population (p = 0.170) or in both subgroups (EWS, p = 0.950; OS, p = 1.000). The other MRI parameters alone or in combination were unreliable and did not improve the results. Comparing diagnostic parameters of FDG PET and local MRI, metabolic imaging showed high superiority in the subgroup of OS patients, while similar results were observed in the population of EWS.
Conclusion
FDG PET appears to be a useful tool for non-invasive response assessment in the group of OS patients and is superior to MRI. In EWS patients, however, neither FDG PET nor volumetry or standardized MRI criteria enabled a reliable response assessment to be made after neoadjuvant treatment.



Similar content being viewed by others
References
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–90.
Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst J, et al. Results of the EICESS-92 Study: two randomized trials of Ewing’s sarcoma treatment—cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol 2008;26:4385–93.
Paulussen M, Ahrens S, Dunst J, Winkelmann W, Exner GU, Kotz R, et al. Localized Ewing tumor of bone: final results of the cooperative Ewing’s Sarcoma Study CESS 86. J Clin Oncol 2001;19:1818–29.
Salzer-Kuntschik M, Brand G, Delling G. Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe 1983;4:135–41. German.
Pan G, Raymond AK, Carrasco CH, Wallace S, Kim EE, Shirkhoda A, et al. Osteosarcoma: MR imaging after preoperative chemotherapy. Radiology 1990;174:517–26.
Holscher HC, Bloem JL, Nooy MA, Taminiau AH, Eulderink F, Hermans J. The value of MR imaging in monitoring the effect of chemotherapy on bone sarcomas. AJR Am J Roentgenol 1990;154:763–9.
Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau AH, et al. Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology 1992;182:839–44.
MacVicar AD, Olliff JF, Pringle J, Pinkerton CR, Husband JE. Ewing sarcoma: MR imaging of chemotherapy-induced changes with histologic correlation. Radiology 1992;184:859–64.
van der Woude HJ, Bloem JL, Holscher HC, Nooy MA, Taminiau AH, Hermans J, et al. Monitoring the effect of chemotherapy in Ewing’s sarcoma of bone with MR imaging. Skeletal Radiol 1994;23:493–500.
van der Woude HJ, Bloem JL, Hogendoorn PC. Preoperative evaluation and monitoring chemotherapy in patients with high-grade osteogenic and Ewing’s sarcoma: review of current imaging modalities. Skeletal Radiol 1998;27:57–71.
Uhl M, Saueressig U, Koehler G, Kontny U, Niemeyer C, Reichardt W, et al. Evaluation of tumor necrosis during chemotherapy with diffusion-weighted MR imaging: preliminary results in osteosarcomas. Pediatr Radiol 2006;36:1306–11.
Brisse H, Ollivier L, Edeline V, Pacquement H, Michon J, Glorion C, et al. Imaging of malignant tumors of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol 2004;34:595–605.
Onikul E, Fletcher BD, Parham DM, Chen G. Accuracy of MR imaging for estimating intraosseous extent of osteosarcoma. AJR Am J Roentgenol 1996;167:1211–5.
Erlemann R, Sciuk J, Bosse A, Ritter J, Kusnierz-Glaz CR, Peters PE, et al. Response of osteosarcoma and Ewing sarcoma to preoperative chemotherapy: assessment with dynamic and static MR imaging and skeletal scintigraphy. Radiology 1990;175:791–6.
Fletcher BD. Response of osteosarcoma and Ewing sarcoma to chemotherapy: imaging evaluation. AJR Am J Roentgenol 1991;157:825–33.
Murphy Jr WA. Imaging bone tumors in the 1990s. Cancer 1991;67(4 Suppl):1169–76.
Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol 2005;15:1658–66.
Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2007;34:463–71.
Weber WA, Wieder H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol Imaging 2006;33 Suppl 1:27–37.
Ott K, Weber WA, Lordick F, Becker K, Busch R, Herrmann K, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2006;24:4692–8.
Ott K, Herrmann K, Lordick F, Wieder H, Weber WA, Becker K, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res 2008;14:2012–8.
Hernandez-Pampaloni M, Takalkar A, Yu JQ, Zhuang H, Alavi A. F-18 FDG-PET imaging and correlation with CT in staging and follow-up of pediatric lymphomas. Pediatr Radiol 2006;36:524–31.
Körholz D, Kluge R, Wickmann L, Hirsch W, Lüders H, Lotz I, et al. Importance of F18-fluorodeoxy-D-2-glucose positron emission tomography (FDG-PET) for staging and therapy control of Hodgkin’s lymphoma in childhood and adolescence—consequences for the GPOH-HD 2003 protocol. Onkologie 2003;26:489–93.
Furth C, Steffen IG, Amthauer H, Ruf J, Misch D, Schönberger S, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol 2009;27:4385–91.
Dutour A, Decouvelaere AV, Monteil J, Duclos ME, Roualdes O, Rousseau R, et al. 18F-FDG PET SUVmax correlates with osteosarcoma histologic response to neoadjuvant chemotherapy: preclinical evaluation in an orthotopic rat model. J Nucl Med 2009;50:1533–40.
Franzius C, Sciuk J, Brinkschmidt C, Jürgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med 2000;25:874–81.
Nair N, Ali A, Green AA, Lamonica G, Alibazoglu H, Alibazoglu B, et al. Response of osteosarcoma to chemotherapy. Evaluation with F-18 FDG-PET scans. Clin Positron Imaging 2000;3(2):79–83.
Hawkins DS, Rajendran JG, Conrad 3rd EU, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer 2002;94:3277–84.
Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad 3rd EU, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828–34.
Schulte M, Brecht-Krauss D, Werner M, Hartwig E, Sarkar MR, Keppler P, et al. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J Nucl Med 1999;40:1637–43.
Hamada K, Tomita Y, Inoue A, Fujimoto T, Hashimoto N, Myoui A, et al. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Ann Nucl Med 2009;23(1):89–95.
Hawkins DS, Conrad 3rd EU, Butrynski JE, Schuetze SM, Eary JF. [F-18]-fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer 2009;115:3519–25.
Piepsz A, Hahn K, Roca I, Ciofetta G, Toth G, Gordon I, et al. A radiopharmaceuticals schedule for imaging in paediatrics. Paediatric Task Group European Association Nuclear Medicine. Eur J Nucl Med 1990;17:127–9.
Stauss J, Franzius C, Pfluger T, Juergens KU, Biassoni L, Begent J, et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging 2008;35:1581–8.
Verstraete KL, Dierick A, De Deene Y, Uyttendaele D, Vandamme F, Roels H, et al. First-pass images of musculoskeletal lesions: a new and useful diagnostic application of dynamic contrast-enhanced MRI. Magn Reson Imaging 1994;12:687–702.
Reddick WE, Wang S, Xiong X, Glass JO, Wu S, Kaste SC, et al. Dynamic magnetic resonance imaging of regional contrast access as an additional prognostic factor in pediatric osteosarcoma. Cancer 2001;91:2230–7.
Bestic JM, Peterson JJ, Bancroft LW. Use of FDG PET in staging, restaging, and assessment of therapy response in Ewing sarcoma. Radiographics 2009;29:1487–500.
Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med 2009;50(9):1435–40.
Franzius C, Bielack S, Flege S, Sciuk J, Jürgens H, Schober O. Prognostic significance of (18)F-FDG and (99m)Tc-methylene diphosphonate uptake in primary osteosarcoma. J Nucl Med 2002;43:1012–7.
Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer 2004;43:723–8.
Lee JA, Kim MS, Kim DH, Lim JS, Yoo JY, Koh JS, et al. Relative tumor burden predicts metastasis-free survival in pediatric osteosarcoma. Pediatr Blood Cancer 2008;50:195–200.
Acknowledgement
This study was financially supported by the Deutsche Krebshilfe e.V. (Grant no. 50-2714-He 1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Timm Denecke and Patrick Hundsdörfer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Denecke, T., Hundsdörfer, P., Misch, D. et al. Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging 37, 1842–1853 (2010). https://doi.org/10.1007/s00259-010-1484-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1484-3