Abstract
Objective
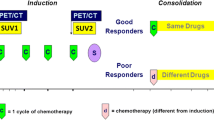
The application of [18F]FDG PET/CT in predicting histologic response to induction chemotherapy in patients with Ewing sarcoma (EWS) has been proposed using the values of pre-post treatment SUVmax as a referral parameter, although with heterogeneous results. The aim of this retrospective study was to evaluate the diagnostic accuracy of [18F]FDG PET/CT volumetric parameters (metabolic tumour volume (MTV) and total lesion glycolysis (TLG)) as compared to SUVmax to predict response to chemotherapy and clinical outcome in patients with localised EWS of bone and soft-tissue.
Methods
Twenty-eight patients with non-metastatic EWS of bone (n = 20) and soft tissues (n = 8) who underwent a [18F]FDG PET/CT scan before (PET1) and after induction chemotherapy (PET2) were enclosed in the analysis. Values of PET metrics (SUVmax, MTV, TLG) at diagnosis and after neoadjuvant chemotherapy as well as the percentage change between PET1 and PET2 (ΔSUV, ΔMTV and ΔTLG) were correlated to histological response and to progression-free survival (PFS).
Results
ΔTLG (cut-off: -60%) is the best predictor for histologic response with 100% sensitivity and 77.8% specificity. MTV1 > 33.4 cm3 and TLG1 > 112 were also associated with a favourable histologic response (sensitivity 80% and specificity 77.8% for both). On multivariate analysis, SUV2 (> 3.3) and ΔTLG (< -18%) were independent predictors of worse PFS.
Conclusions
[18F]FDG PET/CT could accurately predict histologic response to neoadjuvant chemotherapy in patients with EWS, also showing a possible prognostic value for future disease relapse.
Key Points
• The variation of the PET parameter tumour lesion glycolysis (TLG) can predict the histologic response to induction chemotherapy (sensitivity 100%, specificity 77.8%), in patients with Ewing sarcoma.
• The percentage variation of TLG and the value of the SUVmax at PET scan after chemotherapy show a prognostic role for future disease relapse. The combination of both the parameters identifies three prognostic classes of patients with low, intermediate and high risk of disease relapse.




Similar content being viewed by others
Abbreviations
- [18F]FDG PET/CT:
-
[18F]Fluorodeoxyglucose positron emission/computed tomography
- EWS:
-
Ewing sarcoma
- FDG:
-
Fluorodeoxyglucose
- MTV:
-
Metabolic tumour volume
- TLG:
-
Total lesion glycolysis
- ΔMTV:
-
Percentage variation of metabolic tumour volume
- ΔTLG:
-
Percentage variation of total lesion glycolysis
- ΔTV:
-
Percentage variation of tumour volume
References
Gaspar N, Hawkins DS, Dirksen U et al (2015) Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol 33:3036–3046. https://doi.org/10.1200/JCO.2014.59.5256
Ozaki T, Hillmann A, Hoffmann C et al (1996) Significance of surgical margin on the prognosis of patients with Ewing’s sarcoma. A report from the Cooperative Ewing’s Sarcoma Study. Cancer 78:892–900. https://doi.org/10.1002/(SICI)1097-0142(19960815)78:4<892::AID-CNCR29>3.0.CO;2-P
Grünewald TGP, Cidre-Aranaz F, Surdez D et al (2018) Ewing sarcoma. Nat Rev Dis Primers 4:5. https://doi.org/10.1038/s41572-018-0003-x
Bacci G, Ferrari S, Longhi A et al (2003) Therapy and survival after recurrence of Ewing’s tumors: the Rizzoli experience in 195 patients treated with adjuvant and neoadjuvant chemotherapy from 1979 to 1997. Ann Oncol 14:1654–1659. https://doi.org/10.1093/annonc/mdg457
Stahl M, Ranft A, Paulussen M et al (2011) Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 57:549–553. https://doi.org/10.1002/pbc.23040
Rodriguez-Galindo C, Billups CA, Kun LE et al (2002) Survival after recurrence of Ewing tumors: the St Jude Children’s Research Hospital experience, 1979-1999. Cancer 94:561–569. https://doi.org/10.1002/cncr.10192
Bosma SE, Lancia C, Rueten-Budde AJ et al (2019) Easy-to-use clinical tool for survival estimation in Ewing sarcoma at diagnosis and after surgery. Sci Rep 9:11000. https://doi.org/10.1038/s41598-019-46721-8
Wunder JS, Paulian G, Huvos AG et al (1998) The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am 80:1020–1033. https://doi.org/10.2106/00004623-199807000-00011
Picci P, Böhling T, Bacci G et al (1997) Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J Clin Oncol 15:1553–1559. https://doi.org/10.1200/JCO.1997.15.4.1553
Lin PP, Jaffe N, Herzog CE et al (2007) Chemotherapy response is an important predictor of local recurrence in Ewing sarcoma. Cancer 109:603–611. https://doi.org/10.1002/cncr.22412
Whelan J, Le Deley M-C, Dirksen U, et al (2018) High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk Ewing sarcoma: results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol JCO2018782516. https://doi.org/10.1200/JCO.2018.78.2516
Ning MS, Perkins SM, Borinstein SC et al (2016) Role of radiation in the treatment of non-metastatic osseous Ewing sarcoma. J Med Imaging Radiat Oncol 60:119–128. https://doi.org/10.1111/1754-9485.12389
Haveman LM, Ranft A, Vd Berg H et al (2019) The relation of radiological tumor volume response to histological response and outcome in patients with localized Ewing Sarcoma. Cancer Med 8:1086–1094. https://doi.org/10.1002/cam4.2002
Denecke T, Hundsdörfer P, Misch D et al (2010) Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging 37:1842–1853. https://doi.org/10.1007/s00259-010-1484-3
Kubo T, Furuta T, Johan MP et al (2016) Percent slope analysis of dynamic magnetic resonance imaging for assessment of chemotherapy response of osteosarcoma or Ewing sarcoma: systematic review and meta-analysis. Skeletal Radiol 45:1235–1242. https://doi.org/10.1007/s00256-016-2410-y
Hawkins DS, Schuetze SM, Butrynski JE et al (2005) [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 23:8828–8834. https://doi.org/10.1200/JCO.2005.01.7079
Raciborska A, Bilska K, Drabko K et al (2016) Response to chemotherapy estimates by FDG PET is an important prognostic factor in patients with Ewing sarcoma. Clin Transl Oncol 18:189–195. https://doi.org/10.1007/s12094-015-1351-6
Benz MR, Czernin J, Tap WD et al (2010) FDG-PET/CT imaging predicts histopathologic treatment responses after neoadjuvant therapy in adult primary bone sarcomas. Sarcoma 2010:143540. https://doi.org/10.1155/2010/143540
Kim DH, Kim SY, Lee HJ et al (2011) Assessment of chemotherapy response using FDG-PET in pediatric bone tumors: a single institution experience. Cancer Res Treat 43:170–175. https://doi.org/10.4143/crt.2011.43.3.170
Palmerini E, Colangeli M, Nanni C et al (2017) The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas. Eur J Nucl Med Mol Imaging 44:215–223. https://doi.org/10.1007/s00259-016-3509-z
Casali PG, Bielack S, Abecassis N et al (2018) Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv79–iv95. https://doi.org/10.1093/annonc/mdy310
Huang T, Li F, Yan Z et al (2018) Effectiveness of 18F-FDG PET/CT in the diagnosis, staging and recurrence monitoring of Ewing sarcoma family of tumors: a meta-analysis of 23 studies. Medicine (Baltimore) 97:e13457. https://doi.org/10.1097/MD.0000000000013457
Hongtao L, Hui Z, Bingshun W et al (2012) 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: a meta-analysis. Surg Oncol 21:e165–e170. https://doi.org/10.1016/j.suronc.2012.07.002
Gaston LL, Di Bella C, Slavin J et al (2011) 18F-FDG PET response to neoadjuvant chemotherapy for Ewing sarcoma and osteosarcoma are different. Skeletal Radiol 40:1007–1015. https://doi.org/10.1007/s00256-011-1096-4
Bailly C, Leforestier R, Campion L et al (2017) Prognostic value of FDG-PET indices for the assessment of histological response to neoadjuvant chemotherapy and outcome in pediatric patients with Ewing sarcoma and osteosarcoma. PLoS One 12:e0183841. https://doi.org/10.1371/journal.pone.0183841
El-Hennawy G, Moustafa H, Omar W et al (2020) Different 18 F-FDG PET parameters for the prediction of histological response to neoadjuvant chemotherapy in pediatric Ewing sarcoma family of tumors. Pediatr Blood Cancer:e28605. https://doi.org/10.1002/pbc.28605
Hwang JP, Lim I, Kong C-B et al (2016) Prognostic value of SUVmax measured by pretreatment fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with Ewing sarcoma. PLoS One 11:e0153281. https://doi.org/10.1371/journal.pone.0153281
Salem U, Amini B, Chuang HH et al (2017) 18F-FDG PET/CT as an Indicator of Survival in Ewing Sarcoma of Bone. J Cancer 8:2892–2898. https://doi.org/10.7150/jca.20077
Shi J, Yang J, Ma X, Wang X (2020) Risk factors for metastasis and poor prognosis of Ewing sarcoma: a population based study. J Orthop Surg Res 15:88. https://doi.org/10.1186/s13018-020-01607-8
WHO Classification of Tumours Editorial Board (2020) Soft tissue and bone tumours. WHO classification of tumours series, Vol. 3, 5th edn. International Agency for Research on Cancer, Lyon, France. https://publications.Iarc.fr/588
Salah S, Abuhijla F, Ismail T et al (2020) Outcomes of extraskeletal vs. skeletal Ewing sarcoma patients treated with standard chemotherapy protocol. Clin Transl Oncol 22:878–883. https://doi.org/10.1007/s12094-019-02202-y
Acknowledgements
The authors wish to thank Miss Elisa Checcucci and Miss Wioletta Falthyn, respectively, Data Manager and Case Manager of the Disease Management Team (DMT) of bone and soft tissue sarcomas at our Institution, for their invaluable collaboration on patient management and data collection.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr Alessio Annovazzi.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors have significant statistical expertise.
Informed consent
Due to the retrospective design of the study, a formal consent was not required; however, all patients enclosed in the analysis gave permission to use their data in anonymous and aggregate form for research activities, at the time of [18F]FDG PET/CT scan
Ethical approval
The study has been approved by the local institutional ethics committee (prot. no. RS1362/20-2372) and it has been performed in accordance with the ethical standards.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Annovazzi, A., Ferraresi, V., Anelli, V. et al. [18F]FDG PET/CT quantitative parameters for the prediction of histological response to induction chemotherapy and clinical outcome in patients with localised bone and soft-tissue Ewing sarcoma. Eur Radiol 31, 7012–7021 (2021). https://doi.org/10.1007/s00330-021-07841-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07841-w