Abstract
Background
Extracorporeal carbon dioxide removal (ECCO2R) systems have gained clinical appeal as supplemental therapy in the treatment of acute and chronic respiratory injuries with low tidal volume or non-invasive ventilation. We have developed an ultra-low-flow ECCO2R device (ULFED) capable of operating at blood flows comparable to renal hemodialysis (250 mL/min). Comparable operating conditions allow use of minimally invasive dialysis cannulation strategies with potential for direct integration to existing dialysis circuitry.
Methods
A carbon dioxide (CO2) removal device was fabricated with rotating impellers inside an annular hollow fiber membrane bundle to disrupt blood flow patterns and enhance gas exchange. In vitro gas exchange and hemolysis testing was conducted at hemodialysis blood flows (250 mL/min).
Results
In vitro carbon dioxide removal rates up to 75 mL/min were achieved in blood at normocapnia (pCO2 = 45 mmHg). In vitro hemolysis (including cannula and blood pump) was comparable to a Medtronic Minimax oxygenator control loop using a time-of-therapy normalized index of hemolysis (0.19 ± 0.04 g/100 min versus 0.12 ± 0.01 g/100 min, p = 0.169).
Conclusions
In vitro performance suggests a new ultra-low-flow extracorporeal CO2 removal device could be utilized for safe and effective CO2 removal at hemodialysis flow rates using simplified and minimally invasive connection strategies.
Similar content being viewed by others
Background
Mechanical ventilation has long been the standard of care for severe lung failure. A major and paradoxical complication of mechanical ventilation is direct trauma to already ailing lungs caused by over-distention and damage of alveolar tissue due to excessive positive pressures or volumes in the lung [1]. Extracorporeal membrane oxygenation, or ECMO, has more recently become recognized as a last resort option for severe lung failure when mechanical ventilation is failing or is not an alternative. Unlike mechanical ventilation, ECMO performs the function of blood oxygenation and carbon dioxide (CO2) removal independently of the lungs, allowing injured tissue to rest and heal [2]. ECMO is associated, however, with a higher risk of severe complications compared to mechanical ventilation because it requires full circulatory diversion of venous blood to achieve its intended function [3].
The primary complication risks of ECMO are associated with cannulation, exposure of blood to foreign materials, the concomitant requirement for systemic anticoagulation, and the stresses induced by mechanical pumping [4]. The degree of risk associated with these factors correlates with the extracorporeal blood flow rate necessary for treatment [4,5,6]. To provide full extracorporeal oxygenation of venous blood requires circuit flows up to the full cardiac output (4000–7000 mL/min) [5, 7]. In contrast, full metabolic CO2 removal can be achieved at much lower extracorporeal blood flows. CO2 is predominantly carried in the form of highly soluble bicarbonate ion that rapidly restores depleting CO2 as it is eliminated, and the CO2 dissociation curve is essentially linear and does not saturate like the oxyhemoglobin dissociation curve [8,9,10]. These differences also provide the opportunity to augment CO2 removal efficiency with gas exchanger design features aimed at reducing the thickness of the diffusive boundary layer at the gas exchange surface, where gas transport through blood is limited to diffusion [11].
The degree of risk associated with extracorporeal lung support is reduced when lower blood flows are needed to provide clinically meaningful benefit [5]. The ability to efficiently remove CO2 at lower blood flows has motivated use of extracorporeal CO2 removal, or ECCO2R, as an alternative or supplement to mechanical ventilation. The two primary clinical indications where this objective is feasible are acute exacerbations of chronic obstructive pulmonary disease (ae-COPD) and moderate to severe ARDS, where lung protective ventilation strategies are necessary but are unable to maintain safe levels of CO2 removal [12, 13]. ECCO2R was shown to reduce intubation rates in ae-COPD patients failing less-invasive ventilation and assisted in weaning from ventilation [14,15,16,17,18,19]. Hypercapnia was also managed in moderate ARDS patients using ECCO2R to facilitate more protective ventilation strategies by enabling reduction of tidal volumes to ≤ 4 mL/kg without complications [20, 21]. Associated risk remains the primary obstacle of ECCO2R adoption in these indications however. Currently approved ECCO2R systems can operate at blood flows around 500 mL/min, but still require cannula with size greater than 15 Fr [4]. The ability to provide the same levels of CO2 removal at even lower flows will enable the use of smaller catheters that are similar in size to commonly used dialysis catheters that are 9–14 Fr.
We are developing a next-generation ECCO2R device that operates at lower blood flows (250 mL/min) with minimal surface area and clinically significant CO2 removal rates. CO2 removal of 70–160 mL/min has been shown to benefit patients with hypercapnia, which translates to a target CO2 removal rate of ≥ 25–35% metabolic CO2 production (~ 200–250 mL/min) at normocapnia (pCO2 = 45 mmHg) [22, 23]. The device also should maintain an optimal degree of fluid washing around the hollow fiber membranes to eliminate regions of stagnation but without causing unacceptable levels of blood cell trauma. We have adapted technology from our intravenous respiratory assist catheter to accomplish these objectives [24, 25]. Using an array of rotating impellers within an annular hollow fiber membrane bundle, high fluid velocities, and improved blood flow distribution enhances gas exchange. This paper reports on the design and bench testing of an ultra-low-flow ECCO2R device (ULFED) utilizing the rotating impeller concept. In vitro gas exchange and hemolysis were evaluated.
Methods
Device description
The ultra-low-flow ECCO2R device (ULFED) (Fig. 1) contains six rotating impellers fixed on a rigid stainless steel driveshaft (3/16 in. diameter [4.76 mm]). A stainless steel safety coil surrounding impellers (diameter 14.4 mm) protects a 30-cm-long polypropylene (PP) fiber bundle (300 μm diameter, x30-240; Membrana Celgard, Wuppertal, Germany) with total surface area 0.42 m2. The bundle/impeller assembly is housed in cylindrical acrylic tubing (inner diameter 1.375 in. [34.9 mm]) with 1/4 in [6.35 mm] inflow/outflow ports for a total priming volume of 240 mL. Impellers (Fig. 2) were fabricated from a hydrophobic epoxy resin (Watershed XC11122; DSM Somos, Sittard) using stereolithography (SLA). Impellers measured 4 mm in length with a maximum outer diameter of 11.7 mm. Impellers are designed to only generate flow radially in/out of the surrounding fiber bundle to avoid perturbing circuit blood flow rates.
The impeller drive shaft extends out of the blood pathway and is sealed (400054; SKF, Gothenburg, Sweden) and supported by bearings (Ceramic R3; Ortech, Inc., Sacramento, CA). An external DC brushless servomotor (4490 H 048B; MicroMo Electronics, Inc., Clearwater, FL) drives shaft rotation. Saline is continuously infused along the shaft at 30 mL/h to lubricate and protect the seal and bearing from blood backflow up the driveshaft. Heparin was added to the saline infusion (20 U/mL) to maintain anticoagulation levels in the blood for consistency across test circuits. The shaft is supported distally using a custom pivot bearing (ceramic pin (MSC Industrial Supply, Melville, NY) nested in an ultra-high molecular weight polyethylene cup (UHMWPE; Orthoplastics, Lancashire, UK)) shown in Fig. 2.
Gas exchange
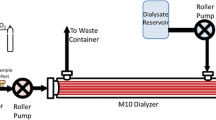
CO2 removal performance of the ULFED prototype was evaluated in a single-pass flow loop (Fig. 3) at a hemodialysis blood flow rate of 250 mL/min. The evaluations followed ISO 7199:2009 standards for gas exchange testing in blood oxygenators [26]. Filtered and heparinized bovine blood (20 U/mL) was collected fresh from the slaughterhouse the day of testing. The fluid circuit consisted of a centrifugal blood pump (BPX-80; Medtronic, Minneapolis, MN), a commercial oxygenator (Affinity NT; Medtronic, Minneapolis, MN), two blood reservoirs connected in parallel, and the ULFED. Blood continuously recirculated at 4500–5500 mL/min while gas tensions were balanced by the commercial oxygenator to normocapnic venous conditions (pCO2 = 45 ± 5 mmHg) using a N2/CO2/O2 gas mixture. Blood temperature was maintained at 37 ± 1 °C with a heat exchanger integrated into the commercial oxygenator. Flow recirculated only to and from the primary reservoir during balancing, while secondary reservoir tubing remained clamped. Gas levels in the recirculating loop were monitored with a blood gas analyzer (RapidPoint 405; Siemens, Erlangen, Germany) until venous conditions were reached. The loop was then converted to single-pass mode for data collection by diverting flow to the secondary reservoir and clamping the bypass tubing in parallel to the ULFED. Measurements were collected at rotation speeds from 0 to 5000 RPM once all measured parameters remained stable for ≥ 2 min. The order of tested rotation speeds was randomized, and a minimum of two measurements were collected at each rotation speed. Blood flow rate was continuously monitored with an ultrasonic flow probe (Transonic Systems, Ithaca, NY).
Pure O2 sweep gas was pulled through fibers counter-current to blood flow at 8.0 L/min by a sealed vacuum pump (N811 KV.45P; KNF Neuberger, Trenton, NJ) and was regulated with a thermal mass flow controller (GR-116-1-A-PV-O2; Fathom Technologies, Georgetown, TX). The fraction of CO2 in outlet sweep gas \( \left({F}_{{\mathrm{CO}}_2}\right) \) was measured by a gaseous CO2 analyzer (WMA-4; PP Systems, Amesbury, MA) and was used to calculate total CO2 removal (\( {V}_{{\mathrm{CO}}_2} \)) together with the STP-corrected sweep gas flow rate \( \left({Q}_{\mathrm{OUT}}^{\mathrm{STP}}\right) \) according to Eq. 1.
\( {V}_{\mathrm{C}{\mathrm{O}}_2} \) was normalized to our target inlet pCO2 of 45 mmHg to reduce variability in measurements associated with small fluctuations in gas inlet conditions (± 5 mmHg) according to Eq. 2.
\( pC{O}_2^{\mathrm{INLET}} \)was measured from a fresh blood sample immediately prior to each data point. Three identical ULFED prototypes were fabricated for repeatability testing, but gas pathway failure in one device limited gas exchange testing to two devices. Total ULFED gas exchange is reported as average and standard deviation of the CO2 removal rates of both prototypes at each rotation speed.
In vitro hemolysis testing
Filtered and heparinized bovine blood (20 U/mL) was collected fresh from the slaughterhouse the day of testing per ASTM standards (F1841–97) [27]. The gas exchange loop was modified for hemolysis testing by removing the bypass tubing parallel to the ULFED, the commercial oxygenator, the secondary reservoir, and the ULFED gas pathway components. The ULFED was evaluated in two circuits so that overall hemolysis reflected that of clinical setups. A reasonable cannula for ECCO2R at the target 250 mL/min of blood flow (13 Fr Avalon Elite DLC 10013; Maquet, Rastatt, Germany) and a pediatric centrifugal pump (PediMag; Thoratec, Pleasanton, CA) were selected for the ULFED “standard circuit”. Blood (1000 mL) was continuously recirculated for 3 h. The reservoir was submerged in a heated water bath to maintain a circuit temperature of 37° ± 1 °C. ULFED rotation was set to the minimum speed necessary where CO2 removal did not differ significantly from the maximum rate achieved. The second ULFED circuit (“dialysis configuration”) evaluated performance using a hemodialysis controller roller pump (Prisma; Baxter, Deerfield, IL) and cannula. A larger bore 14-Fr, 15-cm dialysis cannula (AK-22142-F; Teleflex, Morrisville, NC) was used in the second circuit due to availability of parts recommended for the target blood flows.
A control circuit (“Minimax”) was tested to evaluate ULFED hemolysis against an approved low-flow blood oxygenator (Minimax Plus; Medtronic, Minneapolis, MN). Blood flow in the control loop was maintained at the minimum rate necessary (1250 mL/min) to match ULFED CO2 removal performance according to the manufacturer [28]. Pump (BP-50; Medtronic, Minneapolis, MN) rotation speed in the loop was maintained at 2100–2200 RPM against 180 mmHg to simulate inclusion of cannula recommended for use at the target blood flows (14 Fr Biomedicus 96820-014 venous, 12 Fr Biomedicus 96820-012 arterial) [29, 30]. Pressure against the pump was adjusted using a Hoffman clamp on ULFED outlet tubing and was continuously monitored with a differential fluid pressure transducer (PX771-025DI; Omega Engineering, Inc., Stamford, CT) across the pump. All other components and conditions were consistent between circuits. All three ULFED prototypes fabricated for gas exchange testing were evaluated for hemolysis in both circuit configurations, as the gas pathway failure observed in one prototype did not interfere with hemolysis testing.
Samples were drawn every 30 min to measure hematocrit (HCT) and plasma-free hemoglobin (pfHb). Plasma was isolated from whole blood in two centrifuge spins (15 min at 0.8g, 10 min at 7.2g), and absorbance at 540 nm was measured spectrophotometrically (Genesys 10S UV-Vis; Thermo Scientific, Waltham, MA). PfHb concentration was calculated from absorbance using a standard curve developed from a linear-fit of serially diluted whole blood with 100% hemolysis versus absorbance [31].
The normalized index of hemolysis (NIH) was calculated for circuit comparisons:
Where NIH = normalized index of hemolysis in grams of hemoglobin released into the blood per 100 L of flow through the circuit (g/100 L); ΔpfHb = increase in pfHb over the sampling time interval (g/L); V = circuit volume (L); HCT = hematocrit (%); Δt = sampling time interval (min); Q = average blood flow rate (L/min). A time-of-therapy normalized index was also calculated, since the NIH equation does not reflect total hemolysis returned to a patient in the context of treatment duration. Flow rate normalization in the NIH equation is eliminated in the new therapeutic index of hemolysis (TIH) calculation to indicate the total grams of hemoglobin released to the blood per 100 min of therapy (g/100 min):
Statistics
All statistical comparisons were conducted in SPSS (IBM, Armonk, NY). A one-way ANOVA with Tukey HSD post hoc testing was used to compare removal rates at each RPM after data satisfied assumptions of homogeneity of variance, normality, and independence. Comparisons were used to identify the minimum speed necessary to achieve statistically equivalent performance to the maximum CO2 removal rate. The determined rotation speed was used for subsequent hemolysis testing. Mean NIH values were compared using a one-way ANOVA with Tukey HSD post hoc test after satisfying relevant assumptions. TIH data violated the assumption of homogeneity of variance via Levene’s test, and means were compared with Welch’s F test. Subsequent Games-Howell post hoc tests were used for between-group comparisons of means. All comparisons of means were considered significant at the level p < 0.05.
Results
In vitro gas exchange
Figure 4 shows the raw and normalized CO2 removal rates of the ultra-low-flow CO2 removal device (ULFED) as a function of impeller rotational speed. A sharp increase in CO2 removal occurred between 0 and 2000 RPM before subsequently leveling off at higher rotation speeds. A maximum normalized CO2 removal rate of 75.1 ± 1.1 mL/min was achieved at 5000 RPM. Normalized performance at 4000 RPM did not differ significantly from the maximum rate however (74.1 ± 3.3 mL/min, p = 0.99).
In vitro hemolysis
Measured rates of pfHb accumulation were highly linear over testing periods as shown in Fig. 5 (ΔpfHb versus elapsed time R 2 > 0.95 in all tests). Table 1 shows the calculated hemolysis indices for the ULFED (at 4000 PRM) and the control device. NIH values for the standard-ULFED circuit (0.78 ± 0.19 g/100 L), dialysis-ULFED circuit (1.55 ± 0.03 g/100 L), and the control circuit (0.11 ± 0.01 g/100 L) each differed significantly from one another (ANOVA p < 0.001, all group-wise comparisons p < 0.001). The TIH value of the standard-ULFED (0.190 ± 0.041 g/100 min) did not differ significantly from the control circuit (0.123 ± 0.013 g/100 min; Welch’s test p < 0.001, group-wise p = 0.169). The hemolysis using dialysis circuit components (0.386 ± 0.010 g/100 min) was significantly greater than both other test groups (each p < 0.05). Average hematocrit at each sampling interval is shown in Fig. 5 (right). Hematocrit decreased by ~ 1.5–2.5% from baseline over test periods, which is consistent with dilution due to saline infusion.
Discussion
Supplementing respiration by removing CO2 independent of the lungs can improve outcomes for patients at risk of requiring or already receiving invasive mechanical ventilation. We developed the ultra-low-flow ECCO2R device (ULFED) to operate at blood flow rates consistent with renal hemodialysis to simplify circuit management and minimize invasiveness of CO2 removal. In vitro CO2 removal rates up to 74 mL/min at 4000 RPM were achieved by the ULFED with minimal cell trauma (therapeutic index of hemolysis, TIH = 0.19 g/100 min) at blood flows consistent with dialysis (250 mL/min).
CO2 removal systems used in conjunction with non-invasive or protective ventilation strategies have been shown to correct pCO2 and pH in hypercapnic patients with removal rates equivalent to ~ 25–35% of the metabolic CO2 production (~ 200–250 mL/min) [22, 23]. CO2 removal at these levels prevented intubation in patients with ae-COPD failing or unresponsive to non-invasive ventilation [14, 16, 17, 32]. Partial respiratory assistance has also aided weaning from ventilation [14, 33, 34] and allows reduction of ventilator tidal volumes to ultra-protective levels (3–4 mL/kg) [21, 35, 36]. The ULFED exceeded these CO2 removal rates by eliminating ~ 30–37% of the metabolic CO2 production at normocapnic test conditions (inlet pCO2 = 45 mmHg). Gas exchange will also increase proportionally with pCO2 in hypercapnic patients, where CO2 removal up to 50% or more of metabolic production can be required.
Efficient gas exchange in the ULFED minimizes necessary fiber surface area and enables clinically significant CO2 removal rates at hemodialysis blood flows. Pump-less arteriovenous CO2 removal (AVCO2R) requires dual cannulation (13–19 Fr) for circuit flows of 600–2000 mL/min that is shunted between the femoral artery and vein through a 1.3-m2 oxygenator [36,37,38]. A newer integrated pump-oxygenator system uses a rotating core to generate active mixing to improve gas exchange up to 60% with a 0.59-m2 bundle [39]. Comparatively lower flows (350–500 mL/min) are possible in the simplified veno-venous circuit, but CO2 removal decreases with blood flow (~ 50 mL/min at 300 mL/min blood flow) and connection requires 15.5-Fr cannulation [4, 39]. Developing systems combine existing oxygenators with dialysis controllers targeting even lower flows (200–300 mL/min) to minimize cannulation invasiveness (≤ 14 Fr). These systems utilize larger surface area gas exchangers (≥ 1 m2) to improve performance [32, 40] or target lower CO2 removal using smaller pediatric oxygenators (40–55 mL/min with a 0.3-m2 bundle in pigs with PaCO2 > 80 mmHg) [41]. Approaches to enhance CO2 removal such as bicarbonate dialysis [42], blood acidification [43], electrodialysis [44], plasma recirculation [45], and fiber enzyme coatings [46] are also being explored to reduce necessary blood flows for treatment.
The rotating impellers in the ULFED enhance gas transfer by generating an “active mixing” effect in the fiber bundle that improves convective mixing at gas exchange surfaces [24, 25, 47, 48]. Computational simulations have indicated development of continuously recirculating flow pathways in/out of the fiber bundle with impeller mixing [25]. Blood is pumped radially outward through the bundle by impellers, then pulled back into the bundle toward low-pressure regions in the gaps between impellers before converging onto the impeller blade and cycling through the bundle again. Increasing flow velocity past gas exchange surfaces is a well-established mechanism for improving transfer efficiency by diminishing the thickness of the surface diffusive boundary layer [49]. This facilitates replenishment of gases to the membrane surface to maximize the concentration gradients spanning fiber walls. Recirculating flow also maintains a high level of washing in the bundle that eliminates regions of stagnation where thrombus formation may otherwise occur at low blood flows.
The measured rate of hemolysis in the standard-ULFED circuit was comparable to a clinically approved oxygenator circuit. Two indices of red cell trauma are reported here that indicate the rate of pfHb accumulation over time, the key difference being how time is reported. A major limitation of the NIH calculation is that hemolysis is normalized for blood flow rate, but operating flow rate is ultimately irrelevant. Two systems intended for use at 5000 mL/min versus 250 mL/min that cause equivalent rates of total cell damage would differ in NIH by a factor of 20, despite returning an equal number of pfHb species to a patient. As a result the NIH calculation is bias against low-flow devices. The TIH calculation removes the flow rate normalization and provides a clinically relevant time-of-therapy rate of hemolysis. The limitation of both indices however is that no reliable benchmark threshold values have been validated for low-flow devices against in vivo performance to our knowledge. More information or in vivo testing is therefore necessary to make conclusions regarding acceptability of the dialysis-ULFED performance. No difference in hemolysis was observed between the control and standard-ULFED circuits, so we expect in vivo hemolysis to be acceptable in this configuration.
Anticoagulation of saline infused to the ULFED may have clinical implications with extended use. Heparin levels in the saline infusion line were chosen primarily to avoid dilution of circuit anticoagulation for consistency between tests. Approved blood pumping devices utilizing saline-lubricated seals anticoagulate infusion lines at higher rates [50], while others do not require anticoagulation [51]. Elimination or minimization of local anticoagulation from the ULFED will be investigated in future prototypes utilizing a saline infusion line.
Conclusions
Evidence continues to grow that ECCO2R can effectively prevent intubation, facilitate earlier extubation, or allow reduction of ventilator settings in hypercapnic respiratory failure. The ULFED eliminates clinically significant levels of CO2 from blood with acceptable hemolysis at hemodialysis blood flows, making minimally invasive dialysis connection strategies and simplified management possible for ECCO2R. Future work may focus on in vivo validation of benchtop performance or improvements to the ULFED aimed at simplifying the design, such as sealing the blood compartment with a magnetically coupled driveshaft that would obviate the driveshaft seal and saline infusion.
Abbreviations
- ECCO2R:
-
Extracorporeal carbon dioxide removal
- ECMO:
-
Extracorporeal membrane oxygenation
- NIH:
-
Normalized index of hemolysis
- pfHb:
-
Plasma-free hemoglobin
- TIH:
-
Therapeutic index of hemolysis
- ULFED:
-
Ultra-low-flow ECCO2R device
References
Ricard J-D, Dreyfuss D, Saumon G (2003) Ventilator-induced lung injury. Eur Respir J 22:2s–9s. https://doi.org/10.1183/09031936.03.00420103
Peek GJ, Mugford M, Tiruvoipati R et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363. https://doi.org/10.1016/S0140-6736(09)61069-2
Terragni PP, Maiolo G, Tenaglia T et al (2011) Extracorporeal CO2 removal and O2 transfer: a review of the concept, improvements and future development. Trends Anaesth Crit Care 1:123–127. https://doi.org/10.1016/j.tacc.2011.03.002
Lund LW, Federspiel WJ (2013) Removing extra CO2 in COPD patients. Curr Respir Care Rep 2:131–138. https://doi.org/10.1007/s13665-013-0057-x
Schmidt M, Tachon G, Devilliers C et al (2013) Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 39:838–846. https://doi.org/10.1007/s00134-012-2785-8
Oliver WC (2009) Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 13:154–175. https://doi.org/10.1177/1089253209347384
MacLaren G, Combes A, Bartlett R (2012) Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 38:210–220. https://doi.org/10.1007/s00134-011-2439-2
Silverthorn DU (2007) Human physiology: an integrated approach, 4th edn. Pearson Education, Inc., San Francisco
Kolobow T, Gattinoni L, Tomlinson T et al (1977) The carbon dioxide membrane lung (CDML): a new concept. ASAIO J 23:17–21
Loeppky JA, Luft UC, Fletcher ER (1983) Quantitative description of whole blood CO2 dissociation curve and Haldane effect. Respir Physiol 51:167–181. https://doi.org/10.1016/0034-5687(83)90038-5
Cove M, MacLaren G, Federspiel W, Kellum J (2012) Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care 16:232–230. https://doi.org/10.1186/cc11356
Brower RG, Matthay MA, Morris A et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Lightowler JV, Wedzicha JA, Elliott MW, Ram FSF (2003) Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 326:185
Burki NK, Mani RK, Schmidt W et al (2013) A novel extracorporeal CO2 removal system: results of a pilot study in COPD patients with hypercapnic respiratory failure. Chest 143:678–686. https://doi.org/10.1378/chest.12-0228
Bonin F, Sommerwerck U, Lund LW, Teschler H (2013) Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg 145:e43–e44. https://doi.org/10.1016/j.jtcvs.2013.01.040
Kluge S, Braune SA, Engel M et al (2012) Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38:1632–1639. https://doi.org/10.1007/s00134-012-2649-2
Braune S, Sieweke A, Brettner F et al (2016) The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med 42(9):1437–1444. https://doi.org/10.1007/s00134-016-4452-y
Mani RK, Schmidt W, Lund LW, Herth FJF (2013) Respiratory dialysis for avoidance of intubation in acute exacerbation of COPD. J Novemb 59:675–678. https://doi.org/10.1097/MAT.0000000000000004
Cole S, Barrett N, Glover G et al (2014) Extracorporeal carbon dioxide removal as an alternative to endotracheal intubation for non-invasive ventilation failure in acute exacerbation of COPD. J Intensive Care Soc 15:1–3
Terragni PP, Del Sorbo L, Mascia L et al (2009) Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 111:826–835. https://doi.org/10.1097/ALN.0b013e3181b764d2
Fanelli V, Ranieri MV, Mancebo J et al (2016) Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care 20:36. https://doi.org/10.1186/s13054-016-1211-y
Trahanas JM, Lynch WR, Bartlett RH (2016) Extracorporeal support for chronic obstructive pulmonary disease: a bright future. J Intensive Care Med. https://doi.org/10.1177/0885066616663119
Morelli A, Del Sorbo L, Pesenti A et al (2017) Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med. https://doi.org/10.1007/s00134-016-4673-0
Mihelc KM, Frankowski BJ, Lieber SC et al (2009) Evaluation of a respiratory assist catheter that uses an impeller within a hollow fiber membrane bundle. ASAIO J 55:569–574
Jeffries RG, Frankowski BJ, Burgreen GW, Federspiel WJ (2014) Effect of impeller design and spacing on gas exchange in a Percutaneous respiratory assist catheter. Artif Organs 38:1007–1017. https://doi.org/10.1111/aor.12308
(2009) ANSI/AAMI/ISO 7199:2009 -- Cardiovascular implants and artificial organs—blood-gas exchangers (oxygenators)
ASTM F1841–97 (2005). Standard practice for assessment of hemolysis in continuous flow blood pumps
(2008) Medtronic Minimax Plus, Instructions For Use: M932349A001 Rev. 1.0
Svitek RG, Smith DE, Magovern JA (2007) In vitro evaluation of the TandemHeart pediatric centrifugal pump. ASAIO J 53:747–753. https://doi.org/10.1097/MAT.0b013e318154ca74
Paulsen MJ, Orizondo R, Le D et al (2013) A simple, standard method to characterize pressure/flow performance of vascular access Cannulas. ASAIO J 59:24–29. https://doi.org/10.1097/MAT.0b013e3182746401
Dobrovolskaia MA, Clogston JD, Neun BW et al (2008) Method for analysis of Nanoparticle hemolytic properties in vitro. Nano Lett 8:2180–2187. https://doi.org/10.1021/nl0805615
Del Sorbo L, Pisani L, Filippini C et al (2015) Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 43:120–127. https://doi.org/10.1097/CCM.0000000000000607
Abrams DC, Brenner K, Burkart KM et al (2013) Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10:307–314. https://doi.org/10.1513/AnnalsATS.201301-021OC
Hermann A, Staudinger T, Bojic A et al (2014) First experience with a new miniaturized pump-driven venovenous extracorporeal CO2 removal system (iLA Activve): a retrospective data analysis. ASAIO J 60:342–347. https://doi.org/10.1097/MAT.0000000000000073
Moss CE, Galtrey EJ, Camporota L, et al (2016) A retrospective observational case series of low flow veno-venous extracorporeal carbon dioxide removal use in patients with respiratory failure: ASAIO J 1. doi: https://doi.org/10.1097/MAT.0000000000000386
Bein T, Weber-Carstens S, Goldmann A et al (2013) Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS. Intensive Care Med 39:847–856. https://doi.org/10.1007/s00134-012-2787-6
Zimmermann M, Bein T, Arlt M et al (2009) Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care 13:R10
Bein T, Weber F, Philipp A et al (2006) A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 34:1372–1377. https://doi.org/10.1097/01.CCM.0000215111.85483.BD
Jeffries RG (2014) In vitro and seven-day chronic in vivo evaluation of the hemolung adult CO2 removal system for pediatric respiratory support
Karagiannidis C, Kampe KA, Sipmann FS et al (2014) Veno-venous extracorporeal CO2 removal for the treatment of severe respiratory acidosis: pathophysiological and technical considerations. Crit Care 18:R124. https://doi.org/10.1186/cc13928
Godet T, Combes A, Zogheib E et al (2015) Novel CO2 removal device driven by a renal-replacement system without hemofilter. A first step experimental validation. Anaesth Crit Care Pain Med 34:135–140. https://doi.org/10.1016/j.accpm.2014.08.006
Cressoni M, Zanella A, Epp M et al (2009) Decreasing pulmonary ventilation through bicarbonate ultrafiltration: an experimental study. Crit Care Med 37:2612–2618. https://doi.org/10.1097/CCM.0b013e3181a5668a
Zanella A, Mangili P, Redaelli S et al (2014) Regional blood acidification enhances extracorporeal carbon dioxide removal: a 48-hour animal study. Anesthesiol Febr 120:416–424. https://doi.org/10.1097/ALN.0000000000000099
Zanella A, Castagna L, Salerno D et al (2015) Respiratory electrodialysis: a novel, highly efficient, extracorporeal CO2 removal technique. Am J Respir Crit Care Med 192:719–726. https://doi.org/10.1164/rccm.201502-0289OC
Gramaticopolo S, Chronopoulos A, Piccinni P et al (2010) Extracorporeal CO2 removal—a way to achieve ultraprotective mechanical ventilation and lung support: the missing piece of multiple organ support therapy. Contrib Nephrol 165:174–184. https://doi.org/10.1159/000313757.
Arazawa DT, Kimmel JD, Federspiel WJ (2015) Kinetics of CO2 exchange with carbonic anhydrase immobilized on fiber membranes in artificial lungs. J Mater Sci Mater Med 26:5525. https://doi.org/10.1007/s10856-015-5525-0
Hewitt TJ, Hattler BG, Federspiel WJ (1998) A mathematical model of gas exchange in an intravenous membrane oxygenator. Ann Biomed Eng 26:166–178
Makarewicz AJ, Mockros LF, Anderson RW (1993) A pumping intravascular artificial lung with active mixing. ASAIO J 39:M466–M469
Wickramasinghe SR, Semmens MJ, Cussler EL (1992) Mass transfer in various hollow fiber geometries. J Membr Sci 69:235–250. https://doi.org/10.1016/0376-7388(92)80042-I
Lee Y, Weeks PA (2015) Effectiveness of protocol guided heparin anticoagulation in patients with the TandemHeart percutaneous ventricular assist device. ASAIO J Am Soc Artif Intern Organs 61:207–208. https://doi.org/10.1097/MAT.0000000000000176
Jeffries RG, Mussin Y, Bulanin DS et al (2014) Pre-clinical evaluation of an adult extracorporeal carbon dioxide removal system with active mixing for pediatric respiratory support. Int J Artif Organs 37:888–899. https://doi.org/10.5301/ijao.5000372
Acknowledgements
This work was supported by the grants 5R01HL070051-08 and 5R01HL117637-05 from the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NHLB. This work was also supported by the University of Pittsburgh’s McGowan Institute for Regenerative Medicine. Funding for R. Garrett Jeffries was partially provided by an NIH training grant (T32-HL076124) for the University of Pittsburgh Cardiovascular Bioengineering Training Program (CBTP).
Funding
RJ, BF, and WF received research support from the NIH and NHLBI (5R01HL070051-08 and 5R01HL117637-05), and the McGowan Institute for Regenerative Medicine. RJ was partially funded by an NIH training grant (T32-HL076124).
Availability of data and materials
All relevant data is presented within the text to support findings. No additional data was submitted with the article.
Author information
Authors and Affiliations
Contributions
RJ, BF, and WF contributed to the conception and design of the study and analysis and interpretation of data. RJ, LL, and WF contributed to the drafting the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
WF is an equity holder and Head of the Scientific Advisory Board, for which he receives compensation, at ALung Technologies, which is commercializing an artificial lung device independent of the device described in this article. LL is a full-time employee at ALung Technologies. RJ and BF have no relevant competing interests to report.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jeffries, R.G., Lund, L., Frankowski, B. et al. An extracorporeal carbon dioxide removal (ECCO2R) device operating at hemodialysis blood flow rates. ICMx 5, 41 (2017). https://doi.org/10.1186/s40635-017-0154-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-017-0154-1