Abstract
Background
Critically ill patients with acute respiratory distress syndrome and acute exacerbations of chronic obstructive pulmonary disease often develop hypercapnia and require mechanical ventilation. Extracorporeal carbon dioxide removal can manage hypercarbia by removing carbon dioxide directly from the bloodstream. Respiratory hemodialysis uses traditional hemodialysis to remove CO2 from the blood, mainly as bicarbonate. In this study, Stewart’s approach to acid-base chemistry was used to create a dialysate that would maintain blood pH while removing CO2 as well as determine the blood and dialysate flow rates necessary to remove clinically relevant CO2 volumes.
Methods
Bench studies were performed using a scaled down respiratory hemodialyzer in bovine or porcine blood. The scaling factor for the bench top experiments was 22.5. In vitro dialysate flow rates ranged from 2.2 to 24 mL/min (49.5–540 mL/min scaled up) and blood flow rates were set at 11 and 18.7 mL/min (248–421 mL/min scaled up). Blood inlet CO2 concentrations were set at 50 and 100 mmHg.
Results
Results are reported as scaled up values. The CO2 removal rate was highest at intermittent hemodialysis blood and dialysate flow rates. At an inlet pCO2 of 50 mmHg, the CO2 removal rate increased from 62.6 ± 4.8 to 77.7 ± 3 mL/min when the blood flow rate increased from 248 to 421 mL/min. At an inlet pCO2 of 100 mmHg, the device was able to remove up to 117.8 ± 3.8 mL/min of CO2. None of the test conditions caused the blood pH to decrease, and increases were ≤0.08.
Conclusions
When the bench top data is scaled up, the system removes a therapeutic amount of CO2 standard intermittent hemodialysis flow rates. The zero bicarbonate dialysate did not cause acidosis in the post-dialyzer blood. These results demonstrate that, with further development, respiratory hemodialysis can be a minimally invasive extracorporeal carbon dioxide removal treatment option.
Similar content being viewed by others
Background
Mechanically ventilating patients with acute respiratory distress syndrome (ARDS) can cause additional lung damage [1]. For ARDS patients, it is recommended to ventilate in accordance with lung protective ventilation (LPV) settings, tidal volumes limited to 6 mL/kg, and plateau pressures limited to 30 cmH2O [2]. Although an ARDS Network clinical trial demonstrated LPV reduced mortality by 8.8% [2], more recent studies have shown that these settings may still cause ventilator-induced lung injury (VILI) [3, 4]. To further reduce mortality, tidal volumes less than 6 mL/kg have been proposed [5]. Several studies have demonstrated the safety and feasibility of ultra-protective lung ventilation when used in conjunction with an extracorporeal CO2 removal (ECCO2R) device [5, 6].
In addition to ARDS, chronic obstructive pulmonary disease (COPD) patients frequently develop hypercapnia during acute exacerbations. Hypercapnia has long been recognized as a marker of poor prognosis in patients with COPD [1], with those requiring mechanical ventilation being particularly challenging to wean [7, 8]. A method to remove CO2 in these patients may obviate the need for mechanical ventilation altogether [9, 10]. When combined with the ARDS population, there is a significant unmet need for a simple, minimally invasive therapy to remove CO2.
Extracorporeal carbon dioxide removal is a technology that involves removal of blood from the patient, which is then pumped through an artificial lung (oxygenator membrane) where CO2 is removed and the decarboxylated blood is subsequently returned to the patient [1]. Conventional ECCO2R requires blood flow rates exceeding 1 L/min, as well as large surface areas for gas exchange in the artificial lung, both of which introduce important limitations. High flow rates require placement of large cannulas, which risk vessel injury and require considerable expertise to place [11]. Large surface areas in artificial lungs demand the use of anticoagulation, and bleeding complications have been high in previous ECCO2R trails [12]. In an attempt to mitigate some of these problems, low flow ECCO2R devices have been introduced, but they may take up to 24 h to control CO2 levels [10], and it has not yet been demonstrated in a clinical trial if these devices remove enough CO2 to support critically ill patients; however, several case reports and single site pilot studies have shown this [9, 10, 13–15].
A different approach involves using hemodialysis to remove CO2 in the form of bicarbonate. In respiratory hemodialysis, blood is passed through a dialyzer and bicarbonate is transferred from the blood to the dialysate based on a bicarbonate concentration difference between the two fluids. This is analogous to the movement of waste products in conventional hemodialysis. The similarities between respiratory and conventional hemodialysis provide additional benefits over membrane lung ECCO2R, including clinician familiarity, availability of dialysis equipment in intensive care units, and a reduced risk of air embolism. Bicarbonate removal, however, has proven challenging due to the development of metabolic acidosis, despite attempts to mitigate the acidosis by replacing the bicarbonate with bases, such as sodium hydroxide, and TRIS [1, 16, 17].
The attempt to “replace” bicarbonate, in these early experiments, reflects conventional acid-base teaching, where pH is dependent on, among other things, bicarbonate concentration. However, 30 years ago, Stewart proposed an alternative model of acid-base physiology based around the important observation that water has a dissociation constant and the principles of electrical neutrality [18]. This approach provides modeling advantages over the traditional acid-base approaches [19] by treating bicarbonate as a dependent anion. Stewart proposed that any compound, in solution, must satisfy both the principles of electrical neutrality and the respective dissociation constant, including water itself, where the dissociation constant (K’w) is equal to the product of hydrogen and hydroxyl ion concentrations ([H] × [OH]). Using simple algebra, Stewart showed plasma pH is dependent on the strong ion difference (SID) of the solution, the partial pressure of CO2 (pCO2), and the total concentration of weak acids (A tot). Similarly, the final bicarbonate concentration is dependent on the same variables, i.e., the concentration of bicarbonate does not predict pH, rather the SID, pCO2, and A tot, determine the final bicarbonate concentration. Therefore, a bicarbonate-free dialysis solution should remove bicarbonate from plasma, and allow it to return to a normal physiological pH as long as the strong ion difference and A tot are maintained. In this manuscript, we explore whether ECCO2R, in the form of respiratory hemodialysis, is feasible without decreasing blood pH using a custom dialysate developed using Stewart’s model.
Methods
Construction of zero bicarbonate dialysate
The electrolyte composition of the dialysate was determined using a published acid-base model, pHorum [20]. This model uses the physicochemical approach to acid-base balance, which is based around the Stewart equation [21] shown below [22].
K c is the combined equilibrium and solubility constant for CO2 (2.45 × 10−11 mol2/(L2*mmHg)), K 3 is the second dissociation constant of carbonic acid (5.76 × 10−11 mol/L), K w is the autoionization constant of water applied to plasma (2.39 × 10−14 mol2/L2), K a is the weak acid dissociation constant (1.77 × 10−7 mol/L), and A tot is the total concentration of weak acids [23].
Using this model, one can predict the pH of blood under a range of circumstances. For the purposes of designing the dialysate, we determined the ideal electrolyte composition of blood that will maintain pH within the normal range after removal of bicarbonate and carbon dioxide. Physiologically, normal electrolyte concentrations were used as the starting inputs for the model. Table 1 below shows the analysis results, which represents the ideal electrolyte composition for blood exiting the dialysis filter following bicarbonate removal, assuming the bicarbonate concentration is <10 mmol/L and pCO2 is <20 mmHg. Since the sieving coefficient is close to 1.0 for most of these electrolytes, we used these concentrations to construct the dialysate. In order to maximize bicarbonate removal in our experiments, the bicarbonate concentration was kept at 0 mmol/L in the dialysate solution. The final pH of the modeled dialysis solution is 10, despite targeting a physiological SID, because the dialysate does not contain protein. The dialysate does not contain calcium to allow for the dialysate to be compatible with regional citrate anticoagulation. Furthermore, at a pH of 10 or more, there is a risk the calcium may precipitate out of solution.
In vitro CO2 removal
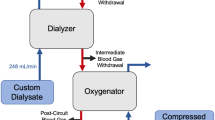
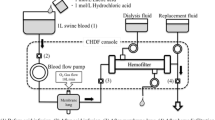
Gas exchange was tested in a single-pass system (Fig. 1). The blood side of the system consisted of a 6-L reservoir bag, a Medtronic Affinity oxygenator (Medtronic, Minneapolis, MN) used to control inlet pCO2 and heat the blood to 37 °C, and a Gambro M10 dialyzer (Gambro, Lyon, Paris; surface area 0.04 m2). The dialysis side of the system consisted of a 6-L reservoir bag submerged in a 37 °C water bath. The zero bicarbonate dialysate used is previously described. Masterflex L/S roller pumps (Cole Palmer Instrument Co, Vernon Hills, IL) were used to control blood and dialysate flow rates.
The blood used was same day bovine or porcine blood collected from a local slaughterhouse (Thoma Meat Market, Saxonburg, PA). The blood was heparinized at a concentration of 10 IU/mL and filtered using a 40-μm pore size filter (Pall Biomedical, Inc., Fajardo, PR). Gentamicin was added at a concentration of 0.1 mg/mL to prevent infection. The blood was diluted using 1× phosphate-buffered solution so that the final hemoglobin concentration was 12 ± 1 g/dL and 5% dextrose in 0.45% NaCl (Baxter Healthcare Corp., Deerfield, IL) was added for a final glucose concentration of 100–300 mg/dL.
The benchtop parameters were scaled down based on the surface area ratio of the M100:M10 dialyzers (0.9/0.04), hence a scale factor of 22.5. The Gambro M100 dialyzer would be the dialyzer used in the scaled up system. Blood flow rates were set to 18.7 mL/min (421 mL/min scaled up) and 11 mL/min (248 mL/min scaled up), and dialysis flow rates were varied between 2.2 and 20 mL/min (49.5–450 mL/min scaled up). Blood gases and pH were measured at the inlet and outlet of the dialyzer and analyzed using a Rapid Point 405 Blood Gas Analyzer with Co-oximetry (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). The targeted inlet pCO2 was 50 ± 5 mmHg.
Total CO2 removal rate was calculated from the change in pCO2 and bicarbonate concentration from the inlet to the outlet of the dialyzer, according to Eq. 2.
∆pCO2 = change in pCO2 across the dialyzer (mmHg), ∆HCO3 = change in actual HCO3 concentration across the dialyzer (mmol/L), K s = CO2 solubility constant in blood (0.0307 mmol/mmHg*L) [24], Q b = experimental blood flow rate (L/min), and V m = molar volume at STP (22.4 mL/mmol). Bicarbonate concentration was calculated using the Henderson-Hasselbalch equation.
Results
CO2 removal rates measured from respiratory dialysis are shown in Fig. 2 are presented, by a dual axis, in terms of actual and scaled up (actual multiplied by the 22.5 scale factor) CO2 removal rates. At an inlet pCO2 of 50 mmHg and a blood flow rate of 18.7 mL/min (421 mL/min scaled up), the maximum CO2 removal rate was 3.5 ml/min (78 mL/min scaled up), a 24% increase compared to a blood flow rate of 11 mL/min (248 mL/min scaled up) at the same conditions (p = 0.048). The effect of doubling the inlet pCO2, 50 mmHg to 100 mmHg, was also evaluated at a blood flow rate of 11 mL/min (248 mL/min scaled up) (Fig. 2). There was an 85% increase in CO2 removal between the two inlet pCO2 conditions.
In vitro CO2 removal rates. The dual axes show experimental results, as well as the expected values when the system is scaled up by a factor of 22.5. The expected scaled up values are in gray, while the actual values are in black. Triangle symbols: Q b = 11 mL/min (248 mL/min scaled up), pCO2, inlet = 100 mmHg; diamond symbols: Q b = 18.7 mL/min (421 mL/min scaled up), pCO2, inlet = 50 mmHg; square symbols: Q b = 11 mL/min (248 mL/min scaled up), pCO2, inlet = 50 mmHg. Single asterisk indicates p < 0.05 between different blood flow rates (diamond and square symbols). Double asterisks indicate p < 0.05 between different inlet pCO2 values (triangle and square symbols)
The blood pH change across the dialyzer is shown in Table 2. No decreases in pH were noted and the largest increase in blood pH was 0.08. This occurred when the inlet pCO2 was 100 mmHg. At 50-mmHg inlet pCO2, the greatest increase in pH was 0.07 and occurred at a blood flow rate of 11 mL/min and a dialysate flow rate of 16 mL/min. At no point did pH fall as a result of bicarbonate removal with the respiratory dialysis procedure.
Discussion
Respiratory hemodialysis, in contrast to traditional ECCO2R using a hollow fiber membrane, is an attractive treatment option for patients with hypercarbic respiratory failure as intensive care units already have dialysis equipment available, clinical staff are familiar with its use, lower flow rates, and much reduced air embolism risk. This study demonstrates the use of a novel zero bicarbonate dialysate in a bench scale respiratory hemodialysis system. When the data is scaled up to an adult hemodialyzer, 62–78 mL CO2/min was removed without changing blood pH.
The inlet pCO2 was doubled, from 50 to 100 mmHg at a constant blood flow rate, in this experiment to evaluate how the CO2 removal rates will scale up when the entire system, including dialyzer surface area and flow rates, is scaled up. In the case of the scaled down system tested here, doubling the amount of available CO2, accomplished by doubling the inlet pCO2, resulted in nearly a doubling of the CO2 removed, on average an 87% increase in CO2 removal across the range of dialysate flow rates tested. Thus, when the system is scaled up, the scaled up flow rate will deliver 22.5 times more CO2 to 22.5 times more dialyzer surface area available for gas exchange, and therefore, the CO2 removal rate should increase by the same factor.
Based on contemporary understanding of acid-base balance, previous work by several groups have attempted to replace the bicarbonate with NaOH, Tris, and organic ions [16, 17], believing this would maintain physiological pH. However, these attempts proved unsuccessful, resulting in hemolysis, elevated pulmonary artery pressure, and metabolic acidosis, among other negative effects. Stewart’s approach to acid-base chemistry, used to design the dialysate in this study, places the SID and A tot as the most important determinants of pH, whereas bicarbonate concentration is dependent on CO2, SID, and A tot, and therefore does not need to be replaced as along as the strong ion concentration and A tot is preserved. The change in blood pH from the inlet to the outlet of the dialyzer is statistically significant for nearly all of the conditions. However, an in vivo respiratory hemodialysis study done by Zanella et al. showed an increase in blood pH of 0.04 between the inlet and outlet of the dialyzer with no reported negative effects [25].
It has been recently demonstrated that CO2 removal rates of 40–80 mL/min maintain normocapnia in ARDS patients treated with protective ventilation and partial ECCO2R at blood flow rates of 400–500 mL/min [5, 26]. The CO2 removal rates achieved in the work described here (63–78 mL/min scaled up) are within clinically useful ranges, and the device was operated at lower blood flow rates (248–421 mL/min scaled up), indicating a higher effectiveness in blood plasma, and a better safety profile. This improved efficiency may reflect the fact that most CO2 is transported as bicarbonate ions (>90%), and the greater effectiveness of bicarbonate dialysate is due to bicarbonate rather than dissolved CO2 being targeted for removal. During ECCO2R using hollow fiber membranes, the CO2 held as bicarbonate must be converted to dissolved CO2 before it can be removed. This conversion limiting CO2 removal rates was demonstrated in a study using hollow fibers immobilized with carbonic anhydrase, to catalyze the conversion of bicarbonate to dissolved CO2, resulting in a 37% enhancement of the CO2 removal rate [27, 28]. Respiratory hemodialysis, by directly removing bicarbonate, is not limited by the conversion of bicarbonate to dissolved CO2 permitting lower blood flow rates yet still able to attain therapeutic results.
Several groups have investigated ways to capitalize on clinician familiarity with dialysis equipment for ECCO2R. The PrismaLung uses the PrismaFlex unit as the blood pump for the Medos Hilite oxygenator and demonstrates the efficacy of using dialysis pumps for ECCO2R. The PrismaLung system removed 40–60 mL/min of CO2 under hypercapnic conditions, at blood flow rates 200–400 mL/min; however, it relies solely on, and is limited by, dissolved carbon dioxide for removal [29]. The respiratory hemodialysis system described in this paper can also use dialysis pumps, but removes 30–58% more CO2 at comparable blood flow rates. Zanella et al. have published several respiratory hemodialysis circuits with dialysate recirculation [25, 30]. These systems have used either a membrane lung with a lactic acid infusion or an electrodialysis unit as secondary CO2 removal devices in the dialysate. The reported CO2 removal rates from the dialysate were 86 and 91 mL/min at a blood flow rate of 250 mL/min, respectively [25, 30].
We recognize that intermittent hemodialysis conditions are not designed for treatment longer than a few hours and a single-pass dialysate may lead to loss of essential minerals, micro-nutrients, hormones, and drugs from the plasma. To prevent this, a less permeable dialyzer could be used or the dialysate could be recycled in a closed loop. The closed loop approach would use a secondary CO2 removal device, such as a bubble oxygenator or membrane lung, to remove CO2 from the dialysate post-dialyzer and drive the bicarbonate concentration back down towards zero. This approach would be attractive because it would recycle the dialysate and reduce cost. Work on dialysate recirculation by Zanella, previously described, demonstrates therapeutic CO2 removal rates and demonstrates that dialysate recirculation for respiratory hemodialysis is possible.
Another limitation of the current bench experiments was the necessity of adjusting dialysate pH to obtain usable measurements with our laboratory blood gas analyzer. For this purpose, hydrochloric acid was added. As a result, the final chloride concentration exceeded 116 mmol/L (Table 1). Consequently, although pH increased slightly in the post-dialyser plasma (Table 2), it did not return to physiologically normal levels of 7.35–7.45, since the chloride resulted in a metabolic acidosis. It should be highlighted, however, that this small pH increase, despite removal of bicarbonate, proving the concept that bicarbonate can be removed without further lowering pH. In future work, we plan to define the safe pH boundaries of our dialysate and target the final dialysate pH accordingly, while limiting chloride, and accomplishing CO2 removal with restoration of a physiological pH. Importantly, bicarbonate removal resulted in lower CO2 levels and was achieved at much lower blood flows than those required for conventional ECCO2R. At these low blood flow rates, about 10% of cardiac output, blood exiting the dialyzer would be significantly diluted by venous blood in circulation.
Conclusions
ECCO2R by continuous hemodialysis is feasible in a bench model of hypercarbic respiratory acidosis without worsening blood pH. There is a critical need for a simple, minimally invasive ECCO2R system. Our respiratory dialysis approach promises to fulfill this need, which may have application in a wider cohort of patients including hypercarbic respiratory failure due to asthma, COPD, and restrictive lung disease as well as enable ultra-protective lung ventilation in ARDS.
Abbreviations
- ∆HCO3 :
-
Change in HCO3 concentration across the dialyzer
- ∆pCO2 :
-
Change in pCO2 across the dialyzer
- ARDS:
-
Acute respiratory distress syndrome
- A tot :
-
Total concentration of weak acids
- CO2 :
-
Carbon dioxide
- COPD:
-
Chronic obstructive pulmonary disease
- ECCO2R:
-
Extracorporeal CO2 removal
- HCO3 - :
-
Bicarbonate ion
- IHD:
-
Intermittent hemodialysis
- K 3 :
-
Second dissociation constant of carbonic acid
- K a :
-
Weak acid dissociation constant
- K c :
-
Combined equilibrium and solubility constant for CO2
- K w :
-
Autoionization constant of water applied to plasma
- LPV:
-
Lung protective ventilation
- pCO2 :
-
Partial pressure of CO2
- Q b :
-
Experimental blood flow rate
- S a :
-
Surface area adult dialyzer
- S f :
-
Surface area experimental dialyzer
- SID:
-
Strong ion difference
- Tris:
-
Tris(hydroxymethyl)aminomethane
- VILI:
-
Ventilator-induced lung injury
- V m :
-
Molar volume
References
Cove M, McLaren G, Federspiel W, Kellum J (2012) Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care 16:232–241
ARDSNetwork (2000) Ventilation with lower tidal volumes as compared with traditional tidal volume for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Terragni P, Rosboch G, Tealdi A et al (2007) Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175:160–166
Grasso S, Stripoli T, De Michele M et al (2007) ARDSnet ventilatory protocol and alveolar hyperinflation role of positive end-expiratory pressure. Am J Respir Crit Care Med 176:761–767
Terragni P, Del Sorbo L, Mascia L et al (2009) Tidal volume lower than 6 ml/kg enhances lung protection. Anesthesiology 111:826–835
Fanelli V, Ranieri M, Mancebo J et al (2016) Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress syndrome. Crit Care 20:1–7
Hilbert G, Gruson D, Portel L et al (1998) Noninvasive pressure support ventilation in COPD patients with postextubation respiratory insufficiency. Eur Respir J 11:1349–1353
Nava S, Rubini F, Zanotti E et al (1994) Survival and prediction of successful ventilator weaning in COPD patients requireing mechanical ventilation for more than 21 days. Eur Respr J 7:1645–1652
Bonin F, Sommerwerck U, Lund L, Teschler H (2013) Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg 145:e43–e44
Burki N, Mani R, Herth F et al (2013) A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 143:678–686
Baker A, Richardson D, Craig G (2012) Extracorporeal carbon dioxide removal (ECCO2R) in respiratory failure: an overview, and where next? J Intensive Care Soc 13:232–237
Morris A, Wallace C, Menlove R et al (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149:295–305
Engel M, Albrecht H, Volz S (2016) Use of extracorporeal CO2 removal to avoid invasive mechanical ventilation in hypercapnic coma and failure of noninvasive ventilation. J Pulm Respir Med 6:357
Abrams D, Brenner K, Burkart K et al (2013) Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10:307–314
Del Sorbo L, Pisani L, Filippini C et al (2015) Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 43:120–127
Nolte S, Benfer R, Grau J (1991) Extracorporeal CO2 removal with hemodialysis (ECBicCO2R): how to make up for the bicarbonate loss? Int J Artif Organs 14:759–764
Gille J, Saunier C, Schrijen F et al (1989) Metabolic CO2 removal by dialysis: THAM vs NaOH infusion. Int J Artif Organs 12:720–727
Kratz A, Ferraro M, Sluss P, Lewandrowski K (2004) Laboratory reference values. N Engl J Med 351:1548–1563
Morgan TJ (2009) The Stewart approach—one clinician’s perspective. Clin Biochem Rev 30:41–54
Kellum J (2005) The Acid Base pHorum. https://www.ccm.pitt.edu/acid-base-phorum. Accessed 5 Aug 2016
Stewart P (1983) Modern quantitative acid-base chemistry. CA J Physiol Pharmacol 61:1444–61
Ring T (2009) Mixing bicarbonates: dilution acidosis from first principles. Intensive Care Med 35:2183–2184
Doberer D, Funk G, Kirchner K, Schneeweiss B (2009) A critique of Stewart’s approach: the chemical mechanism of dilutional acidosis. Intensive Care Med 35:2173–2180
Arthurs G, Sudhakar M (2005) Carbon dioxide transport. Crit Care Pain 5:207–210
Zanella A, Mangili P, Giani M et al (2014) Extracorporeal carbon dioxide removal through ventilation of acidified dialysate: an experimental study. J Heart Lung Trans 33:536–541
Rauch S, Roth H, Kuhwald A et al (2015) Case series of protective ventilation for ARDS using partial extracorporeal CO2 removal
Kimmel J, Arazawa D, Ye S et al (2013) Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J Mater Sci Mater Med 24:2611–2621
Arazawa D, Kimmel J, Federspiel W (2015) Kinetics of CO2 exchange with carbonic anhydrase immobilized on fiber membranes in artificial lungs. J Mater Sci Mater Med 26:1–8
Godet T, Combes A, Zogheib E et al (2015) Novel CO2 removal device driven by a renal-replacement system without hemofilter. A first step experimental validation. Anaesth Crit Care Pain Med 34:135–140
Zanella A, Castagna L, Abd El Aziz El Sayed Deab S et al (2016) Extracorporeal CO2 removal by respiratory electrodialysis: an in vitro study. ASAIO J 62:143–149
Funding
The study was supported by ALung Technologies and the Center for Medical Innovation at the University of Pittsburgh (CMI Grant: F_031-2012).
Authors’ contributions
AM performed the gas exchange experiments. WJF contributed to the analysis and interpretation of the results. AS, MEC, and JAK designed the zero bicarbonate dialysate. AM and AS wrote the manuscript, and all authors participated editing of the manuscript. All authors read and approved the final manuscript.
Competing interests
WJF is the head of the scientific advisory board and an equity holder in ALung Technologies.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
May, A.G., Sen, A., Cove, M.E. et al. Extracorporeal CO2 removal by hemodialysis: in vitro model and feasibility. ICMx 5, 20 (2017). https://doi.org/10.1186/s40635-017-0132-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-017-0132-7